Thermodynamics
Thermodynamics
The following text is used only for teaching, research, scholarship, educational use and informative purpose following the fair use principles.
We thank the authors of the texts and the source web site that give us the opportunity to share their knowledge
Physics
Thermodynamics
We have done a lot of work with energy, but the type of energy we’ve dealt with has been mechanical energy. It is time now to look into other types of energy. Thermal energy has to do with the internal energy of a system – the energy of the particles that make a thing up.
Thermal Energy º The total kinetic energy of the particles in a system
The particles that make up a system are the molecules, atoms, or ions that make it up. These particles have kinetic energy – they have motion. In a gas, the particles are free to zoom around and bounce off other particles. In a liquid they also flit about, but they also make weak bonds with each other and tend to clump together. In a solid the particles are bound together with chemical bonds. These bonds are not rigid, however, so the particles can move back and forth. Kind of like they’re connected to each other with springs that allow them to vibrate.
Thermal Definitions: Here are some important definitions:
Thermal Contact º two systems are placed so that they can exchange thermal Energy
Heat º thermal energy transferred from one system to another.
Temperature º average kinetic energy of the particles in a system.
Thermal equilibrium º A static state. Objects in thermal contact reach the same average internal energy state and no longer exchange thermal energy.
Temperature: Instruments that measure temperature are called thermometers. There are several temperature scales that are in use around the world - the Celsius scale, Fahrenheit scale, Kelvin scale, and the Rankine scale are the major ones.
We will make use of the Celsius scale and the Kelvin scale. The other two scales are only used in the US of A, so we can safely ignore them. (Who cares about a country so backward that they don’t even use the metric system?)
The Celsius scale was originally set up to monitor temperatures on the earth’s surface. The zero point on the scale is fixed at the freezing temperature of water and 100° C is the boiling temperature of the good old H2O (at one atmosphere pressure). The zero on this scale is arbitrary and has no physical meaning as a zero value (zero is supposed to be when you have “nothing”, right?)
Temperatures using the Celsius scale are reported as degrees Celsius. One would say, “Hey, the temperature today is twenty-three degrees Celsius!
The Kelvin scale has a true zero, its zero value represents the lowest possible temperature, which is known as absolute zero. Absolute zero represents the minimum possible energy state for matter.
When reporting a Kelvin temperature, one would say, “Hey it’s three hundred and one Kelvins outside! You don’t use the word “degree” with a Kelvin temperature.
The size of the units for each scale are the same.
Absolute zero, which is zero Kelvins, is –273.15°C. The freezing point of water, 0°C is 273.15 K. Converting between the scales is simple, to convert Celsius to Kelvins you just add 273.15 and to convert Kelvins to Celsius you subtract 273.15.
![]()
![]()
When heat is added to a system, the particles gain kinetic energy. Since their average kinetic energy increases, the temperature increases. Since the particles have increased their kinetic energy, they move faster and further. Each particle takes up more room, so the object as a whole expands.
It expands in all directions.
Engineers and architects have to allow for the expansion of materials with temperature increases when they design things – or else the whole thing could break apart on a cold or hot day. Long structures like bridges have expansion joints to allow for the expansion of the structure with changing temperatures.
Many devices make use of the expansion of materials. Bimetallic strips are used to control the operation of cooling or heating devices. A bimetallic strip is made of two metals that are bonded together. Each metal expands a different amount, so one of the metals expands more than the other. This causes the strip to curve when the temperature changes. This can be used to switch electrical circuits on and off, controlling an air conditioner or a heater.
Heat Flow: Heat can flow from one system to another only if there is a temperature difference between the two systems. The greater the difference in temperature, the faster heat will flow.
![]()
![]()
The direction of heat flow is from a high temperature system to a low temperature system. Heat can only flow in this direction. There is no logical reason for this; after all, isn’t it reasonable to think that in the winter heat could flow from the cold outside into the warm inside of your house? It would cut your energy bill. Unfortunately, that will never happen.
Heat flows from the high temperature system to the low temperature system.
When an object absorbs heat, its particles must somehow gain kinetic energy. They do this by absorbing heat. There are three ways that heat can be transferred between systems: conduction, radiation, and convection. The Physics Kahuna hopes that you are familiar with these. This is the kind of stuff that you do in elementary science classes.
Conduction is heat transfer by direct contact between two systems. When you sit on a really hot car seat and burn your hide, you have gained heat via conduction. Heat will flow from the hot system to the cold system until thermal equilibrium is reached. At that point heat will no longer flow.
What happens in conduction is that the particles in the hot object, placed against the cold object, have collisions with the particles in the cooler system. In these collisions, the particles in the high temperature system lose energy and the particles in the low temperature system gain energy. The thermal energy transfers through the cool system via collisions between the particles until the particles in the two systems have the same average kinetic energy. We say that the systems have reached thermal equilibrium when this happens.
Radiation is heat transfer via electromagnetic waves. A great deal of the energy from the sun reaches the earth in the form of electromagnetic waves. They travel through space. When you go outside on a warm spring day and bask in the warmth of the sun, you are absorbing heat that has radiated from the sun. Electric space heaters that have those glowing red heat elements provide most of their heat via radiation in the same way. Most of the heat that you get from a fireplace (or a camp fire for that matter) arrives via radiation.
The electromagnetic waves travel through space and are absorbed by the system, the absorbed energy is converted into the kinetic energy of the particles – makes them move back and forth.
Convection is heat transfer via fluid flow. A fluid absorbs heat at one location and then flows to another place where it transfers the heat it absorbed to some other system. Convection is used to heat homes via forced air furnaces. A large fan blows warm air into the rooms of the house through ducts. Cooler air is drawn in through grates and returned to the furnace for heating.
Ovens do most of their heating via conduction. An element in the bottom of the oven heats air. The heated air then circulates around and around in the oven transferring heat to the food you want to cook.
It is quite common to have multiple forms of heat transfer in a system. Burning gasoline in a car engine heats the engine block by conduction. Water is circulated through water channels in the engine block absorbing heat via conduction. It then carries the heat to the radiator – this is convection. The heat is then transferred from the water to the radiator via conduction. Air circulates through the radiator removing heat via conduction (when the air is in direct contact with the metal fins of the radiator). The heated air then departs, removing the heat from the radiator via convection.
Heat Transfer by Conduction: Materials that allow heat to flow through them easily are called heat conductors, materials which do not allow heat to flow through them are called heat insulators.
Heat conductors are things like metals. Metals are good conductors because of the nature of the chemical bond that binds the atoms together. These bonds are called metallic bonds. The significant thing about the bonds are that some of the electrons of each atom are not bound to any one particular atom – they’re kind of like “community” electrons belonging to everyone. They are very loosely held and can move around throughout the metal. These are known as free electrons. The free electrons carry the heat from one part of the metal to another. They do this via collisions wherein one electron gives some of its energy to another. This can happen very quickly so that the heat transfers quite easily.
Insulators do not have handy little particles that can collide with each other transferring energy from one place to another. There are no free electrons to do this. If it is a solid, then the electrons are going to be tightly held via covalent or ionic bonds.
Gases make very good insulators because there aren’t many particles to carry the energy from one place to another.
Clearly a vacuum would be even better!
Most insulators are actually materials that have lots of little pockets of air (or a fancy gas) in them. The gas slows the flow of heat way down.
Thermos bottles are very interesting. You can put a hot fluid in the thing and it will stay hot or you can put a cold fluid in it and the fluid will stay cold. The question is this, “How does the bottle know what to do?” Maybe a microchip thingee? Hmmm. Well, actually, thermos bottles are very simple devices. The have an external metal or plastic body, inside of this body is a glass bottle. Really good thermos bottles have a vacuum between the outer case and glass bottle. The glass bottle is mirrored. Heat is kept from flowing by the vacuum which prevents conduction and convection. The mirror surface prevents heat flow by radiation – the mirror reflects the electromagnetic waves (infrared waves, right?). So thermos bottles do a pretty good job of blocking the flow of heat coming either into or out of the bottle. So it can keep hot things hot and cold things cold – without microprocessors.
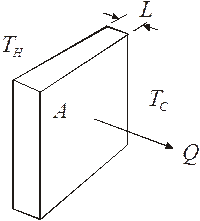 Let us look at a slab of material. One side of the material is at a high temperature and the other side is exposed to a low temperature. Because of the temperature difference, heat will flow through the slab.
Let us look at a slab of material. One side of the material is at a high temperature and the other side is exposed to a low temperature. Because of the temperature difference, heat will flow through the slab.
The rate at which heat is transferred through an object is proportional to the amount of heat that travels through the object divided by the time:
![]()
Where H is the heat flow rate, Q is the quantity of heat transferred, and t is the time.
The amount of heat that makes it through depends on thickness of the substance, the area of the object, and the thermal properties of the material. The thermal properties are expressed in what is called the thermal conductivity of the substance. Each material has its own value for its thermal conductivity. This thermal conductivity is a measure of the ability of a substance to transfer heat. The symbol for thermal conductivity is k. Materials that have a large value for k are good heat conductors, materials with low k values make good insulators.
The heat flow rate for the slab is given by this equation: ![]()
Where H is the heat flow rate, k is the thermal conductivity, A is the area, DT is the temperature difference, and L is the thickness of the material.
H will have units of J/s, J/min, Cal/h, &tc.
Since ![]() and
and ![]() , Then Q = H t so, substituting in for H, we get:
, Then Q = H t so, substituting in for H, we get:
![]()
This would give us the amount of heat flow that would occur in a given time.
So what can we see from this equation?
Well, the heat is directly proportional to the temperature difference and the area. It is indirectly proportional to the thickness of the slab.
Double the area, double the heat flow. Double the temperature difference, double the heat flow. &tc.
However if you double the thickness, the heat flow decreases by two so it is only one half of what it was before.
k can have many different units. Two common units are ![]() or
or ![]()
Generally one looks up the required k values. One could also work them out experimentally.

- A brick oven is 15.0 cm thick. The inside temperature is 350.0 °C. The outside temperature is 25.0 °C. How much heat is lost from one side of the oven in one hour? The side measures 85.0 cm x 60.0 cm. Thermal conductivity of brick? Use 0.71 J/s×m×°C.
![]()

![]()
Mechanical equivalent of heat: One of the great discoveries in physics in the 1800’s was made by James Joule who discovered that heat and mechanical energy were equivalent.
One of the units used to measure heat was the calorie. A calorie is the amount of heat it takes to raise the temperature of one cubic centimeter of water by one degree Celsius. Joule found that heat could be related to mechanical energy.
 Here is a description of Joule’s elegant experiment. An insulated container was filled with water. The temperature of the water could be monitored with a thermometer. In the water tank was a set of paddles that could rotate. A piece of line was attached to the paddles and run over a pulley system. A weight was attached to the line and released. The weight would fall down, causing the paddle to rotate in the water, thus doing work. The temperature of the water increased, indicating that heat had entered the system, even though it was insulated. Joule found that the amount of work done by the weight was equal to the thermal energy that increased the water’s temperature.
Here is a description of Joule’s elegant experiment. An insulated container was filled with water. The temperature of the water could be monitored with a thermometer. In the water tank was a set of paddles that could rotate. A piece of line was attached to the paddles and run over a pulley system. A weight was attached to the line and released. The weight would fall down, causing the paddle to rotate in the water, thus doing work. The temperature of the water increased, indicating that heat had entered the system, even though it was insulated. Joule found that the amount of work done by the weight was equal to the thermal energy that increased the water’s temperature.
The relationship between the calorie and the joule is:
![]()
You’ve heard of calories long before you took chemistry. In these health conscious days, the amount of energy in the food, measured in Calories, is of great concern.
What’s kind of weird is that food calories are different than regular heat calories. Food Calories begin with a capital C and are actually one thousand calories.
1 Cal = 1 kcal
- A serving of fried frog legs provides 876 Cal per serving. If you eat two servings, (a) how many joules of mechanical work must you do to “burn off” the frog leg calories? (b) If you climbed a staircase to work off the frog legs, how high would you have to climb? You have a mass of 58 kg.
(a) 
That’s a lot of joules!
(b) 
That’s a lot of stair climbin’.
Heat and Temperature Change: The heat required to raise the temperature of a substance can be looked at in several ways. The way we look at it in physics is to use a thing called the specific heat.
Specific Heat º heat to raise the temperature of one gram of a substance by one degree Celsius.
The specific heat can be found experimentally, but usually you just look it up in a table. This elegant document has just such a table – the very thing! The Physics Kahuna thinks that it is on the next page or else one of the others.
The heat required to raise a substance’s temperature is given by this equation:
![]()
Q is the heat, m is the mass of the substance, c is the specific heat, and DT is the temperature difference.
- 34 000 J of heat is added to a 2.5 kg sample of water. What is the temperature change that takes place?
We’re not asked to find the final temperature, just the temperature change, so we solve for DT.
![]()

Law of Heat Exchange: When two systems are in thermal contact, heat will flow between them until they reach thermal equilibrium at some equilibrium temperature. The systems will obey the law of heat exchange.
Law of Heat Exchange º The heat lost by the hot system is equal to the heat gained by the cold system.
This means that: QLost = QGained
- A 235 g gold ball at a temperature of 125°C is dropped into an insulated flask of water. The water was initially at a temperature of 22°C. If the equilibrium temperature is 25°C, what is the mass of the water?
![]()

![]()
Table of Specific Heat Values For Common Substances
Substance |
J/kg °C |
Aluminum |
909 |
Beryllium |
182 |
Cadmium |
230.0 |
Brass |
384 |
Copper |
387 |
Germanium |
322 |
Glass |
837 |
Gold |
129 |
Water |
4186 |
Ice |
2090 |
Steam |
201 |
Iron |
448 |
Lead |
128 |
Mercury |
138 |
Silicon |
703 |
Brass |
384 |
Silver |
234 |
- A 158.0 g brass ball at a temperature of 265 °C is dropped into a container containing 550.0 g of water. If the final temperature of the ball is 35.5 °C, what was the initial temperature of the water?

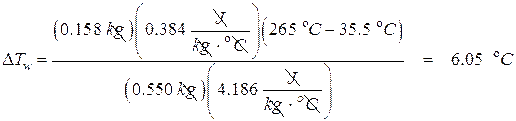
![]()
Phase changes: Phase changes are when a substance goes form one state to another. The states we worry about are: solid, liquid, and gas. So a phase change involves a substance going from one state to another. In physics we are mainly concerned with the energy requirements of the phase change.
Here are the major phase changes:
Melting ® Solid becomes a liquid
Solidification (freezing) ® Liquid becomes a solid
Vaporization ® Liquid becomes a gas
Condensation ® Gas becomes a liquid
Sublimation ® Solid becomes a gas
Deposition ® Gas becomes a solid
Normally when we add heat to a substance its temperature increases. This is what we expect to happen. Heat that does this is called sensible heat. Think of such heat as making sense – you expect heat to change the temperature of a thing.
When a substance changes phase, heat is involved. Some phase changes require heat – you have to add heat to make it happen. These changes are said to be endothermic. Vaporization and melting are endothermic and require the addition of heat.
Exothermic phase changes give off heat. Freezing and condensation are exothermic.
When a substance is changing state, its temperature remains constant. Heat is being added or is leaving, but the heat doesn’t affect the temperature, instead it is involved in either breaking or forming bonds.
Heat added to cause a phase change is called latent heat (latent meaning hidden).
The heat needed to cause vaporization is called the latent heat of vaporization. Usually this is shortened to just “heat of vaporization”. It works for both vaporization and condensation. When the substance condenses after being vaporized, it gives off this heat.
The heat involved in melting or freezing is called the latent heat of fusion. Shortened to “heat of fusion”.
The heat required for a phase change of a substance is given by this equation:
![]()
Q is heat, m is mass, and L is the latent heat for the phase change.
The latent heat of vaporization has this symbol: Lv
The latent heat of fusion has this symbol: Lf
Here’s a handy table of values:
Substance |
Melting Point |
Heat of Fusion |
Boiling Point |
Heat of Vaporization |
Helium |
- 269.65 |
5.23 |
-268.93 |
20.9 |
Nitrogen |
- 209.97 |
25.5 |
-195.81 |
201 |
Oxygen |
-218.79 |
13.8 |
182.97 |
213 |
Ethyl Alcohol |
-114 |
104 |
78 |
854 |
Ammonia |
-75 |
452 |
2 870 |
1 370 |
Water |
0.00 |
333 |
100.00 |
2 256 |
Sulfur |
119 |
38.1 |
444.6 |
326 |
Lead |
327.3 |
24.5 |
1750 |
870 |
Aluminum |
660 |
397 |
2450 |
11 400 |
Silver |
960.80 |
88.2 |
2193 |
2 330 |
Gold |
1063.0 |
64.4 |
2660 |
1 580 |
Copper |
1083.0 |
134 |
1187 |
473 |
Mercury |
-38.87 |
11.8 |
356.58 |
296 |
Tin |
232 |
60.3 |
2270 |
2 200 |
Tungsten |
3410 |
180 |
5927 |
824 kj/mole |
Iron |
1535 |
33.0 |
3000.0 |
6 700 |
Zinc |
419.4 |
96.3 |
907 |
199 |
Heat/Temperature Curves: When a graph is made up temperature vs heat, the curve will look like the generic one below.
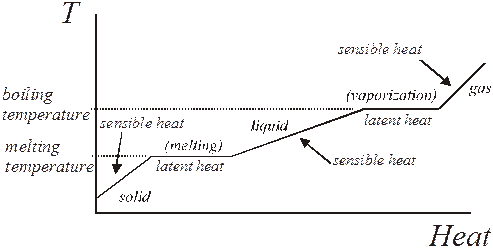
The slopes represent the specific heat of the different phases.
The flat parts of the graph where the temperature does not increase with added heat represent the phase changes. The heat added is latent heat.
Where the graph has a slope, the temperature does increase with energy, so the heat added is sensible heat.
One can find the value of the boiling and melting temperatures by finding the flat areas where the phase changes.
- How much heat is required to melt 2.85 kg of ice at zero degrees Celsius?
This is a simple problem. Just use the phase change equation.

Source : http://teachers2.wcs.edu/high/bhs/mikek/AP%20Physics%20Course%20Notes/Thermodynamics/1%20-%20Thermo.doc
Web site link: http://teachers2.wcs.edu/
Author : not indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly.
Thermodynamics
Thermodynamics
Thermodynamics
This is the right place where find the answers to your questions like :
Who ? What ? When ? Where ? Why ? Which ? How ? What does Thermodynamics mean ? Which is the meaning of Thermodynamics?
Thermodynamics physics notes
Alanpedia.com from 1998 year by year new sites and innovations
Main page - Disclaimer - Contact us