Thermal physics notes
Thermal physics notes
The following text is used only for teaching, research, scholarship, educational use and informative purpose following the fair use principles.
We thank the authors of the texts and the source web site that give us the opportunity to share their knowledge
Physics
Thermal physics notes
PEDAGOGICAL COMMENT FOR THE LEARNER:
Thermal physics differs somewhat from other branches of physics not only in its subject matter (very large systems), but also in its logical structure. There are no grand differential equations (like Maxwell's equations or the Schrodinger equation) that encompass the entire subject. Instead, there are only a few small equations, most of them definitions, together with a bag of tricks forsolving a huge variety of problems. Once the basic concepts are defined, almost everything follows from pure logic.
Because the logic of thermal physics is more important than any particular equations, you should concentrate on the logic, more than the equations, as you study. You'll need to understand, and be able to reproduce, most of the "derivations"; otherwise you will .find it difficult to apply the ideas to new systems that are different from those we discuss in class. (The number of possible applications is so enormous that we'll have time for only a small fraction of them.)
Since the ideas of thermal physics are closely linked to each other, the material of this course will be highly sequential. It is therefore crucial that you follow the activities presented here in the sequence they appeared in the module. If you don't understand something go and refer to the compulsory materials and visit the useful links there in; don't just write it down and hope that you'll figure it out later.
Most important of all, do exercise and self assessments on schedule; don't put it off until the last minute (or later).
Extensive research in recent years has shown that the students who do best in physics (and other subjects) are those who involve themselves actively in the learning process. This involvement can take many forms: writing lots of questions in the margins of the module; asking questions by email; discussing physics in the AVU discussion forums etc.
Key Concepts
Heat: Is the (sum) total internal kinetic energy of all the molecules in a given system of interest. (cup of coffee, bath tub, ocean).
Temperature: Is a measure of the average kinetic energy of the individual particles in an object.the average internal kinetic energy of the molecules in the region of interest.
Heat (internal kinetic energy): always flows from the hotter region (object) to the cooler region (object) for two regions/objects in direct contact (sensible heat exchange).
Fahrenheit scale: Is the temperature scale on which 32 and 212 are the temperatures at which water freezes and boils.
Celsius scale: Is the temperature scale on which zero and 100 are the temperatures at which water freezes and boils.
Kelvin scale: Is the temperature scale on which zero is the temperature at which no more energy can be removed from matter.
Absolute zero: Is the temperature at which no more energy can be removed from matter.
Degree: Is the unit of measurement of temperature.
Calorie: Is the amount of heat required to raise the temperature of one gram of water one degree Celsius.
List of Relevant Readings
- Reference: Douglas D. C. Giancoli Physics for Scientists and Engineers. Vol. 2. Prentice Hall.
Abstract:
Rationale: This reading is a standard text book in many Universities and it provide easy sources of information. The contents have been treated in lucid manner with adequate mathematical support. - Reference: Raymond A. Serway (1992). Physics for Scientists & Engineers. Updated Version.
Abstract:
Rationale: This reading assumes Advanced Level/High School Physics background of the reader. The contents have been treated in lucid manner and it is probably the best at this level.
1: Thermometry
Common-sense notions of heat and temperature are common to all of us. In physics, we need to define the notions of heat, temperature, work, etc. more carefully. Historically, it took a long time to arrive at the proper concept of ‘heat’. The modern concept of heat accepts heat as a form of energy.
An important experiment regarding the concept of heat was due to Benjamin Thomson (also known as Count Rumford) in 1798. He observed that boring of a brass cannon generated a lot of heat, indeed enough to oil water. More significantly, the amount of heat produced depended on the work done but not on the sharpness of the drill. Former views of heat can not explain this observation and the most natural explanation was that heat was a form of energy.
1.1: Thermal Equilibrium
When we put two objects “in contact”, the atoms in those objects can exchange energy. In doing so, some macroscopic (measurable) properties of the objects can change. If we wait long enough, those properties (actually, all the properties that one could measure) will be constant, and at that time we say that the objects are in thermal equilibrium with one another.

Figure 8: A and C are in thermal equilibrium with B. Therefore A and C are at the same temperature
The above statement is known as Zeroth law of thermodynamics. The Zeroth law allows us to know whether objects are at the same temperature, even when we can’t place them in thermal contact and it allows temperature to become a reproducible and quantifiable concept. Notice that body B can be a thermometer as shown in the above diagram.
The Zeroth Law tells us that there is some property that is common to objects in thermal equilibrium. This property is temperature, and so the Zeroth Law is really telling us that temperature is a meaningful concept. Now we need a way to get a quantitative measure of temperature.
1.2: Temperature Scales and Thermometers
As discussed earlier, assigning values of temperature to different bodies is quite arbitrary i.e. a matter of choice provided we ensure that bodies in thermal equilibrium have the same value and those not in thermal equilibrium have un equal values of temperature. Thus, there are several possible temperature scales.
Temperature scales are constructed by choosing two fixed points: one to fix the origin of the scale and the other to fix the size of the unit of the scale. For example in the Celsius scale, the two fixed points are assigned the numbers 0oC and 100oC. On the other hand the two points in the Fahrenheit scale are 32oF and 212oF, thus both the origin and the unit size differ for the two scales.
Any thermometer makes use of some measurable property (called thermometric property) that change with temperature. This could be for example, length, volume, pressure, electrical resistance, thermoelectric e.m.f. radiated power, etc. Suppose we choose electrical resistance as the thermometric property. We first measure the resistance Ro and R100 of the resistance at the two fixed points of the Celsius scale. Then we put the resistance thermometer in contact with the body whose temperature is to be measured, and find its resistance to be Rt. We take the thermometric property (resistance) to change linearly with temperature. The temperature tR of the body is then given by the linear relation:

Figure9: The three most common temperature scales.
A similar procedure can be followed for a thermometer that employs another thermometric porperty say thermoelectric electromotive force.
Let us look at liquid, gas, resistance, thermoelectric thermometers and pyrometers in more detail.
Liquid Thermometers: These thermometers are based on the principle of change in volume with change in temperature. Mercury and alcohol are common liquids used to construct thermometers. There are three scales (i) Centigrade (ii) Reaumer and (iii) Fahrenheit (F). If we have any thermometer marked ![]() for ice point and having
for ice point and having ![]() divisions between boiling and freezing point we have for
divisions between boiling and freezing point we have for ![]() temperature in such a scale
temperature in such a scale
Kelvin or absolute scale ![]() . in general:
. in general:
Gas Thermometers: Gas thermometers are of two types. Constant pressure and Constant volume thermometers.
Constant pressure gas thermometer is based on the principle that pressure remaining constant, the volume of a given gas varies directly as temperature i.e.
where ![]() is known as coefficient of cubical expansion of the gas.
is known as coefficient of cubical expansion of the gas.
Constant volume gas thermometer is based on the principle that when we heat a gas keeping the volume constant its pressure increases and when we cool the gas, its pressure decreases i.e.
where ![]() denote pressure of a gas at constant volume at temperatures
denote pressure of a gas at constant volume at temperatures ![]() respectively
respectively
Platinium Resistance Thermometer: This thermometer works on the principle that electric resistance of metals increases more or less uniformly with temperature. If ![]() and
and ![]() are the resistances of a give wire at
are the resistances of a give wire at ![]() respectively then
respectively then
where ![]() is called temperature coefficient of resistance. The unknown temperature is calculated with the help of following relation
is called temperature coefficient of resistance. The unknown temperature is calculated with the help of following relation
where ![]() is the resistance of the wire at 100
is the resistance of the wire at 100![]()
Thermoelectric Thermometer: This thermometer works on the principle that: when two wires of different metals are joined end to end in a loop and the juncitons are kept at different temperatures, an e.m.f. is produced. The magnitude of this e.m.f. depends upon the difference of the temperature between the hot and cold junctions. By knowing the temperature of one junction and e.m.f. produced, the temperature of unknown body (in contact with other junction) can be known.
Pyrometers:
- Fery’s Total Radiation Pyrometer: This pyrometer works on the principle that a hot body emits radiation and the amount of heat radiated at any time depends upon the temperature of the body. According to Stefan law total amount of heat radiated by a hot body per second per unit area is directly proportional to the fourth power of temperature.
- Disappearing Filament Optical Pyrometer. This works on the principle that on heating the colour of a body changes due to change in temperature and the same colour when bodies have equal temperatures.
Key Concepts
System - In thermal physics the “thing” we’re interested in is usually called the “system” Everything else is called the “surroundings” For example in studying a mixture of ice in a calorimeter the calorimeter the water and the ice together form the system while the ambient air, the table on which the calorimeter is placed form the surrounding. In an automobile engine the burning gasoline and resulting gasses would compose the system. The pistons, block, radiator, outside air, etc. would be the surroundings.
Thermal Equilibrium:- when a system containing two or more objects in contact and at different temperature is left to itself the temperature of each object becomes the same after some time. This situation is known as thermal equilibrium.
Internal Energy - The internal energy of an object or physical system is the sum of the kinetic and potential energies of all the constituent atoms or molecules of the object or system. Potential energy arises from attractive/repulsive forces between atoms or molecules (“balls and springs” model) or from interaction of atoms/molecules with electric/magnetic fields, etc. Relative importance of PE and KE due to mutual interaction depends on phase of matter.
Heat: Is transfer of energy between objects as a result of a temperature difference between them. Notice that temperature of an object is associated with the kinetic energy of the constituent atoms/molecules and this motion of atoms/molecules is random. In other words heat is energy transfer associated with this random motion.
Work: In contrast to heat, which is energy transfer associated with random motion, work is energy transfer associated with directional, ordered motion of atoms/molecules. Compression/expansion of a substance, Application of magnetic field, Application of electric field, Flow of electric current are examples of work.
List of Relevant Readings
- Reference: Kittel C. and Kroemer H., (1980) Thermal Physics, 2nd ed., W. H. Freeman and Co., San Francisco, CA..
Rationale: This classic reference on thermal physics is rcommended for a serious student of physics. The contents have been treated in detail with adequate mathematical support. - Reference: Nelkon & Parker (1995), Advanced Level Physics, 7th ed, CBS Publishers & Ditributer, 11, Daryaganji New Delhi (110002) India. ISBN 81-239-0400-2.
Rationale: This reading provide easy sources of information. The contents have been treated in lucid manner with adequate mathematical support.
2 : Heat
Heat is energy transferred between systems at different temperatures. When talking about heat, we usually use the symbol ![]() . The SI unit for heat is the Joule. However, more common units are Btu (British thermal unit) and calories:
. The SI unit for heat is the Joule. However, more common units are Btu (British thermal unit) and calories:
The internal energy of a body is the sum of the kinetic energies of molecules constituting the body. The potential energy is due to their interaction and the intermolecular energy (i.e. the energy of motion and interaction of atoms, nuclei, ions, etc.). The internal energy of a body depends neither on its motion as a whole nor on its potential energy in an external force field.
In this activity, we shall consider physical phenomena and processes which do not involve a change in the intramolcular energy. Hence, for the sake of convenience and simplicity, we shall treat the internal energy of a body as a sum of the kinetic energies of molecules constituting a substance and the potential energy of their interaction.
The internal energy of a body can be changed as a result of two kinds of effects on the body:
- when work is done on the body (as a result of compression, extension and so on),
- when heat is supplied to the body (heating a gas in a closed vessel, heating a liquid, etc.)
The transport of internal energy from one body to another without work being done by the bodies is called heat transfer. The amount of energy transported from body to body by heat transfer is called the amount of heat.
2.1: Transfer of Heat
The transfer of heat is normally from a high temperature body to a lower temperature body. Heat transfer changes the internal energy of both systems involved.
There are three ways heat energy can move from one place to another. These are conduction, convection and radiation.
Convection (in fluids)
Convection occurs when a gas or liquid has different temperatures within its boundaries. The fluid with the higher temperature is less dense than that with the lower temperature. The cooler fluid will sink and the warmer fluid will rise. This creates a mixing effect that moves heat energy from the bottom to all other areas of the fluid.

Figure 10: when water boils, hot water at the bottom rises to the top and cold water at the top sinks to the bottom.
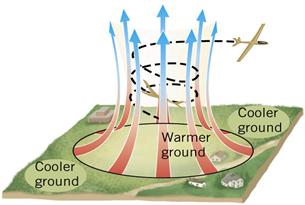
Figure 11:Heat from the sun warms the ground. The atmosphere is heated from the ground by convection
Convection currents in the atmosphere can create a low pressure area. This often happens in cities where the asphalt and concrete get hotter than the surrounding rural areas.
Sometimes the air above a region may be warmer than the air below. This is called an inversion layer because the temperatures are reversed from the normal situation. When this happens, convection does not occur and air pollution increases near the ground under the inversion. Los Angeles is notorius for this effect. Los Angeles lies in a depression between the mountains to the east and a slight ridge near the Pacific Ocean. Air pollution components can be trapped in this bowl shaped area for days.
Conduction (in gases, liquids, and solids)
Conduction is is the process of heat transfer through a substance without any motion of the material as a whole. Conduction can happen in solids, liquids and gasses, but is most noticeable in solids and to a lesser extent in liquids.
The process of conduction occurs as molecules that have been heated gain kinetic energy(speed up their random molecular motion) and collide with adjacent molecules giving them more kinetic energy. These newly energized molecules collide with their cooler neighbors giving them energy and the process is repeated until the entire object has been heated.
Different bodies have different thermal conductivities. Metals are particularly good conductors because they have some electrons that are not bound tightly and are able to transmit this energy of motion more easily than much larger molecules and atoms.

Figure 12: Heat conduction in solids
Thermal conductors allow heat to flow through them freely while thermal insulators do not. The amount of heat energy that flows through a substance depends on several factors. They are time, temperature difference, cross-sectional area, and length(distance).
The equation used to calculate the amount of heat energy that flows during a time ![]() through a bar of cross-sectional area
through a bar of cross-sectional area ![]() with a temperature difference between the two ends of
with a temperature difference between the two ends of ![]() and length
and length ![]() is:
is:
![]() is a constant called the thermal conductivity of the substance and is large for conductors and very small for insulators.
is a constant called the thermal conductivity of the substance and is large for conductors and very small for insulators.
Radiation:
Thermal radiation is the transfer of heat energy by electromagnetic waves. No medium is required. For example, the entire energy received by the Earth from the Sun is transferred by radiation.
All bodies emit some radiation. At lower temperatures, infrared is emitted and can be detected by special optical devices that convert infrared into visible light.
At about 1000 K the red glow associated with hot coals can be seen and at about 1700 K the mixture of frequencies called white light is seen.
The absorption and emission of radiation depends on the nature of the surface. Black, rough surfaces absorb and emit up to 97% of the incident radiation while smooth, silvery surfaces absorb and emit only about 10%.

Figure 13: Heat is better absorbed by black bodies
Lampblack is a substance that is a strong absorber and emitter. In the diagram, most of the energy is absorbed by the lampblack coated block and then reemitted both outward and inward causing the internal temperature to rise. The silver coated block reflects most of the energy that strikes it and does not heat up as rapidly.
That is why we wear dark colors in winter and light colors in the summer. When the insulation for a building is being chosen, the rate of heat flow through the insulator can be calculated using the equation:
![]() is called the
is called the ![]() value of the insulation. It depends on the thickness
value of the insulation. It depends on the thickness ![]() and the conductivity
and the conductivity ![]() k of the insulating material. High
k of the insulating material. High ![]() values mean better resistance to heat flow and may be added to find the total R value of a multilayered wall or other surface.
values mean better resistance to heat flow and may be added to find the total R value of a multilayered wall or other surface.
Figure 14: Heat conduction in solids
2.2: First Law of Thermodynamics
The law of conservation and transformation of energy is fudamental law of nature. It can be formulated as follows:
in all processes ocurring in nature, energy is not created or destroyed. It si transferred from one body to another or converted from one kind to another in eqivalent amounts.
The work ![]() done by the system during a transformation from an initial state to a final state depends on the path taken. The heat Q absorbed by the system during a transformation from an initial state to a final state depends on the path taken. However, the difference Q – W does not depend on the path taken! We define this quantity as the change in internal energy:
done by the system during a transformation from an initial state to a final state depends on the path taken. The heat Q absorbed by the system during a transformation from an initial state to a final state depends on the path taken. However, the difference Q – W does not depend on the path taken! We define this quantity as the change in internal energy:
The internal energy of a system increases if energy is added as heat, and decreases if energy is lost as work done by the system.
2.3: Heat Capacity
Heat capacity ( ), like volume or mass, is a property that depends on the amount of material we are considering. It is the amount of heat that should be supplied to a body in order to raise its temperature by One Kelvin. In the SI units, heat capacity has the dimensions of a joule per kelvin.
), like volume or mass, is a property that depends on the amount of material we are considering. It is the amount of heat that should be supplied to a body in order to raise its temperature by One Kelvin. In the SI units, heat capacity has the dimensions of a joule per kelvin.
A property that only depends on the substance (like density) is specific heat:
where ![]() is the mass of the body.
is the mass of the body.
The specific heat of water is  when a material undergoes a phase transformation (it is melting, or boiling), the temperature will not change, but heat is absorbed (or emitted) in the transformation. The energy per unit mass is called the heat of transformation
when a material undergoes a phase transformation (it is melting, or boiling), the temperature will not change, but heat is absorbed (or emitted) in the transformation. The energy per unit mass is called the heat of transformation  :
:
Heat capacity depends on the condition of heating or cooling a body. For gases, the process of heating (cooling) at constant volume and at constant pressure are of special interest. In former case, we speak of the specific heat at constant volume (![]() ), when the gas does not perform work, and the entire amount of heat supplied to it is spent to raise its internal energy:
), when the gas does not perform work, and the entire amount of heat supplied to it is spent to raise its internal energy: ![]() . In the latter case, the specific heat at constant pressure is meant (
. In the latter case, the specific heat at constant pressure is meant (![]() ). The gas heated at constant pressure expands, and a part of heat supplied to the gas is spent to do the work of expansion:
). The gas heated at constant pressure expands, and a part of heat supplied to the gas is spent to do the work of expansion: ![]() Hence we conclude that
Hence we conclude that ![]() , and the heat capacity at constant pressue is higher thant the heat capacity at constant volume:
, and the heat capacity at constant pressue is higher thant the heat capacity at constant volume: ![]()
2.4: Heat and Work
Work Done in Compression/Expansion
Gas undergoes reversible compression by application of force F to frictionless piston as shown below.

Figure 15: Reversible compression of gas molecules
Assume pressure of gas remains constant
In reality, pressure can/does vary during expansion/compression But pressure can be assumed to be constant in infinitesimal limit:
Calculate W for “large scale”processes by integration
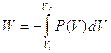

Figure 16:Work done on a gas is the equal to the shaded area.
Work Done is path dependent
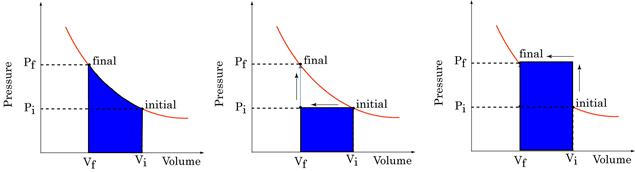
Figure 16: Work done is path dependent
The P-V plots above all represent changes between the same initial and final states. Clearly, the work done in each case is significantly different. Work done depends not only on initial and final states, but also on path taken between them.
Thermodynamic Functions of State
- We’ve seen that work done depends not only on initial and final states, but also on path taken between them
- Similar arguments can be applied to heat added /extracted (SerwayCh. 20).
- Therefore, heat and work do not have definite value corresponding to a particular state of a system.
- Some quantities, eg pressure, volume, temperature and internal energy do have definite values corresponding to a particular state. Changes in these variables are path independent only depend on final and initial states
- Pressure, volume, temperature, internal energy, etc are functions of state: heat and work are not
First Law of Thermodyanics:
KEY CONCEPTS (GLOSSARY)
- Temperature: is a property of an object that indicates in which direction heat energy will flow if the object is placed in thermal contact with another object. Heat energy flows from an object at a higher temperature to the one at a lower temperature. The standard point in temperature measurement is the triple point of water which is arbitrarily assigned a value of 273.6 K. There are three commonly used scales of temperature.
- Centigrade or Celsius Scale

- Fahrenheit Scale

- Reumer Scale

The three scales are related by:

- Zeroth law of Thermodynamics states that: If two bodies
 and
and 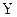 are each separately in thermal equilibrium with another object
are each separately in thermal equilibrium with another object  , then they are in thermal equilibrium with one another.. In the most common case the body
, then they are in thermal equilibrium with one another.. In the most common case the body  is a thermometer.
is a thermometer. - Specific Heat Capacity: Is the heat energy required to raise unit mass of the substance through one Kelvin. The SI units are
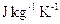
- Calorimetry: is a study concerned with heat measurements
- Specific Latent Heat: Is the quantity of heat required to change unit mass of a solid to liquid, or liquid to gas, without change of temperature. Specific latent heat of fusion is the quantity of heat required to change unit mass of a substance from the solid state to the liquid state without change of temperature. Specific latent heat of vaporization is the quantity of heat required to change unit mass of a substance from the liquid state to the vapor state without change of temperature.
- Newton’s Law of Cooling: States that a body loses heat at a rate proportional to the difference in temperature between the body and the surroundings, provided that the temperature of the body is higher than the temperature of the surroundings.
- Radiation: Is a process by which energy is transmitted by electromagnetic waves.
- First Law of Thermodynamics: States that if a thermally isolated system is brought from one equilibrium state to another, the work necessary to achieve this change is independent of the process used.
- Avogadro’s Number: Is the number of elementary units in one mole of a substance. The Elementary unit may be an atom, molecule, ion, electron, photon, etc. The Avogadro number can be stated as the number of atoms in
 of
of  . The symbol given to Avogadro’s number is
. The symbol given to Avogadro’s number is 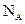 and it is given by
and it is given by 
- State: State describes the physical condition of a given sample of gas. Four quantities describe the state of a gas. These quantities are temperature, pressure, volume and mass.
- Ideal Gas: Is a hypothetical gas with molecules of negligible size that exert no intermolecular forces
- Kinetic Theory of Gases: Is a theory based on the assumption that all matter is made up of very small particles, in constant random motion that experience purely elastic collisions.
- Cycles: Is a process in which a set of operations takes place in some particular order so that at the end of that set of operations initial conditions are restored. In the case of engines the working fuel may be in the form of a gas and after undergoing series of changes in pressure, volume and temperature returns to its original form.
- Carnot Cycle: Is a cycle (of expansion and compression) of an idealized reversible heat engine that does work without loss of heat
- Internal Energy: Is the energy which a system possesses. This energy depends upon the internal state of the system as determined by its pressure, temperature, and composition. The kinetic energy of motion of individual molecules or ions, the kinetic energy and potential energy of electrons and other particles within individual molecules or ions, contribute to the internal energy of the system. Work and heat are means of getting energy in and out of the system and so of changing the internal energy. It can be said that a change of internal energy (U) is equal to the heat (q) added to the system, less the work done (W) by the system:

- Entropy: Is a measure of the amount of disorder in a system; the more disordered the system, the higher the entropy. The disorder may be molecular chaos, e.g. when a liquid changes to a gas at the same temperature the entropy increases because the gas molecules are more disordered than the liquid molecules. Similarly a mixture of two gases has higher entropy than the two separate gases. An entropy change occurs when a system absorbs or evolves heat; the change in entropy is measured as the heat change divided by the temperature at which the change takes place; thus
 , where
, where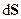 is the entropy change. The entropy of a perfect crystal of each element or compound is given a reference value of zero at absolute zero of temperature
is the entropy change. The entropy of a perfect crystal of each element or compound is given a reference value of zero at absolute zero of temperature - Heat Engine: Is a device that converts thermal energy into other forms of energy such as mechanical, electrical, etc. Heat engines are cyclic devices.
- Heat is absorbed from a high temperature reservoir
- Work is done by the engine
- Heat is expelled by the engine to a lower temperature reservoir
- The engine returns to its initial state
- Second Law of Thermodynamics: States it is impossible for a self acting machine which is working in a cyclic process and is unaided by any external energy to convey heat from a body at a lower temperature to a body at a higher temperature. In other words heat can not flow from a cold body to a hot body without the aid of some external agency.
- Thermodynamic Processes: are changes that taking place in a thermodynamic system. They are of two types:
- Reversible Process: Is a process in which the change can be retraced in the reverse direction. All isothermal and adiabatic changes which are performed very slowly are examples of reversible process if in these changes it is assumed that no heat is lost in friction or to surrounding.
- Irreversible Process: Is a process which can not be retraced . Work done against friction, heat due to flow of current through a conductor are examples of irreversible process.
- Thermodynamic Processes: These can also be classified as:
- Isothermal : Is a process that occurs at constant temperature
- Isobaric : Is a process that occurs at constant pressure
- Isovolumic: Is a process that occurs at constant volume
- Adiabatic : Is a process that occurs without transfer of heat
SELF EVALUATION ASSOCIATED WITH THERMAL PHYSICS:
Evaluate your preparedness to take the module on thermal physics. If you score greater than or equal to 60 out of 75, you are ready to use this module. If you score something between 40 and 60 you may need to revise your school physics on topics of heat. A score less than 40 out of 75 indicates you need to learn school physics.
Try the following questions and evaluate where you are in topics related to thermal physics..
- If the temperature of a patient is 40oC, his temperature on the Fahrenheit scale will be
- 104oF
- 72oC
- 96oC
- 100oC
- A beaker is filled with water at 4oC. At one time the temperature is increased by few degrees above 4oC and at another time it is decreased by a few degrees below 4oC. One shall observe that
- the level remains constant in each case.
- in first case water flows while in second case its level comes down
- in second case water overflows while in first case it comes down
- water overflows in both cases.
- Solids expand on heating because
- kinetic energy of atoms increases
- potential energy of atoms increases
- total energy of atoms increases
- the potential energy versus inter-atomic distance curve is a symmetric about the equilibrium distance and the inter-nuclear distance increases on heating.
- On heating a liquid of coefficient of cubical expansion
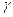 in a container having coefficient of expansion
in a container having coefficient of expansion  , the level of liquid in the container will :
, the level of liquid in the container will :
- rise.
- it is difficult to say
- will remain almost stationary
- fall
- What is the value of absolute zero on the Fahrenheit scale?
- 0oF
- -22oF
- -350oF
- -459.4oF
- Water is used to cool machines. this is mainly because
- It is cheap
- it has high specific heat capacity
- its heat of vaporization is greater than its specific heat capacity
- it is easily available.
- If 50 grams of ice and 50 grams of water are both at 0oC, then it is true that
- the water molecules have a higher average kinetic energy than the ice molecules
- the ice molecules have a higher average kinetic energy than the water molecules
- the water molecules have a higher total potential energy than the ice molecules
- the ice molecules have a higher total potential energy than the water molecules
- Heat travels from one object to another when these objects differ in
- specific heat
- heat capacity
- temperature
- state
- A block of ice at -10oC is slowly heated and converted to steam at 100oC. Which of the following curves represents the phenomenon qualitatively

- The energy radiated by a black body per unit of time is proportional to the absolute temperature raised to the
- first power
- second power
- third power
- fourth power
- A block of metal is heated to a temperature much higher than the room temperatue and allowed to cool in a room free from air currents. Which of the following curves correcltly represents the rate of cooling?

- The heat conducted through a wall in a unit time is
- is directly proportional to the thickness of the wall
- is inversely proportional to the area of the wall
- is directly proportional to the difference in temperature of the two surfaces of the wall
- does not depend on the material in the wall
- An adiabatic expansion of a gas is one in which
- the pressure is kept constant
- the volume is kept constant
- the temperature is kept constant
- it neither loses nor gains heat
- If a Carnot engine operates between temperatures of 27oC and 127oC, its efficiency in percent is
- 20
- 25
- 35
- 50
- Water always boils when
- its temperature reaches 100oC
- its vapor pressure is 1 gram per sq. cm
- its saturated vapor pressure equals the atmospheric pressure
- above which a gas cannot be liquefied
- Equal volumes of gas at the same conditions of temperature and pressure
- contain the same number of molecules
- have the same density
- have the same mass
- have the same ionization potential
- The coefficient of volume expansion (
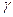 ) surface expansion (
) surface expansion ( ) and linear expansion (
) and linear expansion ( ) are related with each other as
) are related with each other as
- A metallic ball having specific heat 0.22kcal/kgoC weighing 300g is to be converted into liquid at its melting point 660oC. If latent heat of the material of the ball is 76.8klcal/g how much heat is required in k cal for this? Initially the ball is at 20oC.
- 55.3
- 65.3
- 75.3
- 85.3
- Which of the following statement is correct about heat capacity at constant pressure
 and at constant volume
and at constant volume 



- All the above are possible depending upon the situtation
- Which of the following statements is NOT true?
- All reversible engines operaing between same two temperatures have same efficiency
- No engine can be more efficient than carnot engine
- Carnot engine is a reersible engine
- Efficiency of a revesible engined depends on its working substance.

Figure 6: Carnot (1796-1832)
9.2 ANSWER KEY:
- A
- D
- D
- C
- D
- B
- C
- C
- A
- D
- B
- C
- D
- B
- C
- A
- C
- B
- B
- D
Source : http://oer.avu.org/bitstream/handle/123456789/31/Thermal%20Physics.doc?sequence=3
Web site link: http://oer.avu.org/
Author : Tilahun Tesfaye, Ph.D.
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly.
Thermal physics notes
Thermal physics notes
Thermal physics notes
This is the right place where find the answers to your questions like :
Who ? What ? When ? Where ? Why ? Which ? How ? What does Thermal physics notes mean ? Which is the meaning of Thermal physics notes?
Thermal physics notes physics notes
Alanpedia.com from 1998 year by year new sites and innovations
Main page - Disclaimer - Contact us



