Quantum mechanics
Quantum mechanics
The following text is used only for teaching, research, scholarship, educational use and informative purpose following the fair use principles.
We thank the authors of the texts and the source web site that give us the opportunity to share their knowledge
Physics
Quantum mechanics
At the turn of the century, physics was in a terrible fix. What was the problem? Well, it was with the behavior of electrons. Classical physics had gotten quite good at dealing with motion, orbits, &tc. A physicist could sit down and figure out exactly what and where an electron should be doing. Unfortunately, the rotten electrons did not oblige the physicists with doing what it was supposed to be doing.
 Ernest Rutherford pictured the atom as a sort of miniature solar system. The electrons circled the nucleus in orbits, just like the planets circle the sun. According to classical, Newtonian physics, a centripetal force acts on the electron, accelerating it towards the nucleus. This force is caused by the charge difference between the positive nucleus and the negative electron. The problem was this: accelerated electrons emitted light. Thus as the electrons were accelerated towards the nucleus, they should radiate photons of light. This would cause them to lose energy, which would give them a smaller orbit, they would emit light, move closer to the nucleus as their orbit gets smaller, and so on. In about 10-9 seconds the electron would spiral into the nucleus. This meant that atoms as we know them could not exist in a stable state.
Ernest Rutherford pictured the atom as a sort of miniature solar system. The electrons circled the nucleus in orbits, just like the planets circle the sun. According to classical, Newtonian physics, a centripetal force acts on the electron, accelerating it towards the nucleus. This force is caused by the charge difference between the positive nucleus and the negative electron. The problem was this: accelerated electrons emitted light. Thus as the electrons were accelerated towards the nucleus, they should radiate photons of light. This would cause them to lose energy, which would give them a smaller orbit, they would emit light, move closer to the nucleus as their orbit gets smaller, and so on. In about 10-9 seconds the electron would spiral into the nucleus. This meant that atoms as we know them could not exist in a stable state.
Unfortunately for classical physics, but fortunately for the universe, this does not happen. Atoms actually do manage to hang around for times in excess of a billionth of a second. Actually they seem quite capable of hanging around for billions of years. Clearly something was going on with the behavior of electrons that did not fit into classical kinematics.
Black Body Problem: All objects in the universe constantly emit electromagnetic waves. This is a fact of nature. You, as you read this, are quite happily emitting massive numbers of photons from all over your body into the universe. You, blighter that you are, have done this your entire life! People mostly emit long wavelength infrared. The army has these night vision scopes that detect the IR signature given off by humanoids, thus enabling the soldier to “see” in the dark.
The frequency of the emitted electromagnetic waves is a function of temperature – and only temperature. The higher the temperature of the body, the higher the frequency (and the shorter the wavelength) of the emitted electromagnetic wave. It doesn’t matter what the object is made from, for a given temperature it will emit a given frequency of light. Also of course, the higher the frequency of the emitted photons, the greater their energy.
At the turn of the century physicists were looking at “black bodies”. A black body is one of those ideal things that physicists love to invent. (One definition of classical physics is that it deals with elephants with zero volume, no friction, acting independently of gravity. Do you think that is fair?) The black body is defined as an object that absorbs all the light that is incident on it.
Imagine that you have a hollow object – any hollow object will do. Let’s say you have a white plastic ball. It’s white on the outside and white on the inside because it’s made of white plastic. Okay? Got it? Now you make a small hole in the ball. Having done that, you now look into the hole – what do you see? Well, the whole looks black. The inside of the ball looks black, even though we know that it is actually white. So what’s the deal?
The interior of the ball behaves as if were a black body - it absorbs just about all the electromagnetic waves that enter it. Visible light is absorbed and does not get out. In fact the only radiation that does comes out of the hole from the inside of the thing is infrared. The infrared is emitted because of the temperature of the ball. Thus does the stupid hole in the ball behave as a black body.
Stars are considered to be black bodies (even though they certainly aren’t black). Planets can be treated as black bodies as well.

Below is a graph made up of the emissions from a blackbody at three different temperatures. The area under the curve represents the total radiation. Each curve has a peak wavelength – this is the wavelength at which most of the energy is emitted. For 4 000 K you can see that the amount of radiation is much greater than for the lower temperature curves. The 3 000 K curve also has a peak wavelength, but it has a greater value than the one for the higher temperature – the wavelength of the emitted radiation is longer, which means that it has a lower frequency. The 2 000 K curve’s peak wavelength is much smaller in amplitude and longer in wavelength. Its frequency is lower as well.
The general rule is that the intensity and frequency of emitted radiation increases with temperature. This is seen in stars and planets. Planets, which are very cool, don’t even emit visible light, they can only manage infrared. Cool stars give off mostly red light, warmer stars give off yellow light, and the hottest stars give off blue light.
Classical mechanics cannot explain these curves. It works for the long wavelengths, but as the wavelength decreases (and the frequency increases), classical mechanics’ predictions become very bad, very bad indeed. In fact the old theory predicts that the intensity of the emitted radiation will approach infinity as the wavelength nears zero. You can see that this does not happen. The curve shows that as the wavelength gets close to zero, the intensity also approaches zero.
This is one of your basic contradictions. Since classical mechanics cannot explain what actually happens, physicists were forced to abandon the laws of Newton and develop a new theory that would explain the data. The theory that came out of this is known as quantum mechanics.
Max Planck (1858-1947) was a German physicist who spent a great many years trying to puzzle out this problem. Planck was trying to find a fundamental law that would describe the energy emitted by blackbodies. He eventually got the job done, but to get his law to work, he had to assume that the radiation, which everyone knew was a wave, was actually made up of little “packets” of energy (which everyone knew was not true). He called these packets quanta (singular) and quantum (plural). There was no evidence for the quanta, except that the made his law work. Using this cobbled up thing, he was able to explain the blackbody radiation curve and calculate accurate energy for emitted radiation. In other words, the cobbled up thing actually worked! Planck believed that the quanta were merely an artificial, mathematical device without 
reality that just happened to make his equation yield accurate results.
Planck’s equation (which we have seen before – remember that the Physics Kahuna promised to revisit it, well, that time is here) is:
![]()
Here, E is energy, fis the frequency, and h is known as Planck's constant. It’s value is:
![]() or
or ![]()
You will have both of these values when you take the AP Physics test.
- What energy is carried by a photon of electromagnetic radiation that has a frequency of 1.55 x 1017 Hz?

The energy of a photon can also be calculated as a function of wavelength. Wavelength is related to frequency by:
![]() This is the equation for the speed of the wave.
This is the equation for the speed of the wave.
![]()
![]()
You won’t be provided with this equation, so you need to be able to get there on your own.
- A photon has a wavelength of 550 nm. How much energy does this represent in Joules?
![]()

Since the value of Planck’s constant multiplied by the speed of light is itself a constant, we can treat hc as a constant. (Save us some work!) Two such values, using different units, will be provided to you on the AP Test:
![]()
![]()
This makes solving the above problem a lot easier. To wit:
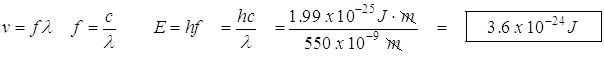
Planck's theory came to be called the quantum theory and proved so important, that it is considered to be a watershed in science. All physics before Planck’s equation is called classical physics and all physics afterwards is known as modern physics.
But what did all this mean?
Momentum and Light: You need to be able to calculate the momentum of a photon as a function of its frequency or wavelength. Okay, let’s do a typical problem.
The momentum for a photon is given by this equation:
![]()
- What is the momentum of a photon that has a wavelength of 455 nm?
![]()
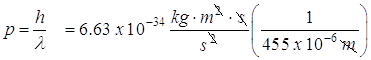

Photons and Power of a Source: Imagine that you have a source of light that is rated at a certain power level. It produces photons of only one frequency. So, how many photons per second would it produce?
This is pretty simple. Power is simply the rate that energy is produced. The energy is in the form of photons. All you have to do is calculate the amount of energy produced in one second. Then determine the amount to energy one photon represents. Then divide the total energy by the energy per photon. This gives you the number of photons in a second. That last part is really just a dimensional analysis problem, ain’t it?
Okay, here’s a problem. Let’s go for it.
- A 505 nm light source produces 0.250 W. How many photons per second does it kick out?
![]()
![]()

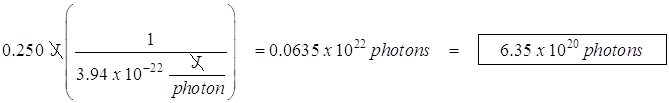
Photoelectric Effect: Towards the end of the 19th Century (in 1887 to be exact) Heinrich Hertz discovered that certain metals would emit electrons when light was incident on them. This was the first instance of light interacting with matter and was very mysterious. In 1905 Albert Einstein, a 3rd Class Technical Expert in the Swiss Patent Office, the obscure physicist (although he was not a physicist at the time, he was a bureaucrat) mentioned before, published a paper which provided the explanation for the effect. The light was actually made up of small particles - Planck’s little bundles of energy he called the quanta. These particles are now called photons.
The surface electrons were bound to the metal with a small amount of energy. Some of the incident photons would enter the surface, smack into atoms of the metal and be totally absorbed. They would give their energy to an electron, which, if the absorbed energy was great enough, could then break free from the atom. You can think of the photoelectric effect as being the result of collisions between photons and electrons, which knock the electrons out of the metal.
The amount of energy binding the electrons to the metal is called the work function. The symbol for this is the Greek letter f.
![]()
Recall that:
![]() This is the energy of the photon.
This is the energy of the photon.
The electron that has been knocked out of the metal has some amount of kinetic energy. This kinetic energy has to be less than the photon’s energy because some of the energy added to the system was used to break the electron free of the metal (this amount of energy is given by the work function). So the photon has to provide more energy than the work function if the electron is to be set free.
The maximum kinetic energy that an electron can have is just the difference between the energy of the work function (the energy that binds the electron to the metal) and the energy of the photon.
![]()
This equation will be provided to you on the AP Physics Test.
Each metal has its own value for the work function. A handsome table of such values for selected metals has been helpfully provided to you.
Work Function for some Different Metals |
|
Metal |
Work Function |
Sodium (Na) |
2.28 |
Aluminum (Al) |
4.08 |
Copper (Cu) |
4.70 |
Cobalt (Co) |
3.90 |
Zinc (Zn) |
4.31 |
Silver (Ag) |
4.73 |
Platinum (Pt) |
6.35 |
Lead (Pb) |
4.14 |
Iron (Fe) |
4.50 |
Dear Doctor Science,
What is a laser beam made of?
-- Lauren Grace from Toledo, OH
Dr. Science responds:
Normal light is comprised of zillions of photons. Laser light is made of futons, which are fat, stuffed photons with a zipper down the side. Some have a foam core and these are often mistakenly referred to as mu mesons, which is just a fancy oriental term for futon. As in retail advertising, Science often gives the prosaic a new name to make it seem like things are really happening when, in fact, everyone is just playing Tetris on their office computers and waiting for lunch.
Wavelength and the Photoelectric Effect: We have an equation that relates the electron’s energy to frequency, but what about the wavelength of the photon? For some reason physicists are very fond of wavelengths and prefer them frequencies.
The frequency and wavelength are related by the speed of light. So when we want to find the value for the frequency we get:
![]()
![]()
we can substitute this into the Planck’s equation and get:

You can then plug this into the photoelectric equation for the energy term:
![]()
Of course, this equation you will not have for the AP Physics test.
What was strange about all this is that the effect is based on the energy of the photons, a function of its frequency or wavelength. The intensity of the light – how “strong” the beam is, does matter, but only if the frequency of the photons is high enough. Photons which have too low a frequency (or too long a wavelength) will not knock any electrons loose no matter how intense the light is. The intensity is really a measure of the number of photons that will be incident on the surface in a given amount of time. So if the frequency is large enough to cause the effect and you increase the intensity, you will increase the photocurrent because there will be more photons hitting the metal to knock loose more electrons.
The kinetic energy of the electrons is also independent of the intensity of the light. More intense light will dislodge more electrons, so the current will increase, but the kinetic energy of the electrons will all be limited to the same value (the maximum kinetic energy).
- What is the maximum kinetic energy of a photoelectron that has been liberated from a silver metal surface by a photon that has a frequency of 3.13 x 1015 Hz?

![]()
- What is the velocity of a photoelectron that has been liberated from a zinc metal surface by a photon that has a wavelength of 275 nm?
Consulting the table, we find that the work function for zinc is 4.31 eV. We can use this and the wavelength of the incident photons to find the kinetic energy of the ejected electrons. We can then solve for their velocity.


![]()
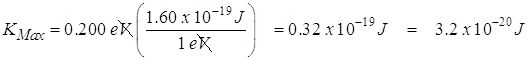
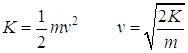


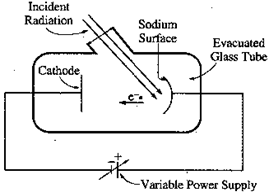
Typical Photoelectric Effect Experiment: A typical laboratory setup for a photoelectric experiment would consist of a metal plate that the light will be incident upon. This plate is called the emitter (E). Across from the emitter is a plate called the collector (C). The emitter is connected to the negative terminal of a variable dc voltage source and the collector is connected to the positive terminal of the source. An ammeter is placed in series with the collector/battery and a voltmeter is placed in parallel with the photoelectric element. A typical circuit is shown below.

Incident light strikes the emitter, which causes photoelectric electrons to be emitted. The electrons are attracted to the positively charged collector and a current is established. We can then measure the current and voltage.
If the wavelength of the incident light is varied, but the intensity of the light is kept constant, then we get a graph of current VS. wavelength that looks like this:
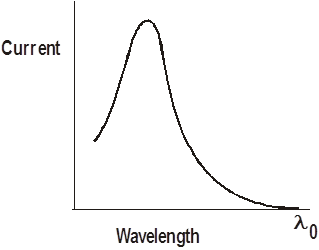
Notice that the current is emitted only for wavelengths less than l0. For longer wavelengths, no current is emitted. These represent photons that don’t have enough energy to knock the electrons out of the metal.
This maximum wavelength, l0 , is called the photoelectric threshold wavelength.
The amazing thing is that the current does not depend on the intensity of the light. This seems to make no sense. You would think that if you made the light brighter, you ought to get a bigger photoelectric current. It’s another instance where classical physics fell apart.
According to classical physics, the incident waves would provide the energy to knock the electrons out of the metal. The greater the intensity of the light, the more electrons ought to be knocked loose. But this didn’t happen. For a great many wavelengths, no photoelectric current would flow no matter how large the intensity.
Current does depend on intensity, but only for wavelengths that cause the photoelectric effect.
Effect of Collector Voltage: If the positive voltage on the collector is increased we soon get a maximum amount of current. If the intensity is increased, we also see an increase in the current. But even for zero voltage on the collector, some current will flow. But what happens if you make the collector’s voltage negative instead of positive?
The electrons will be repelled from the collector. If the voltage is small, some will still make it, but as the voltage gets more negative it becomes harder and harder for the electrons to bridge the gap. More and more are turned away and the current falls off. At some voltage value, none of the electrons make it to the collector and current is zero. This is shown in a graph of Voltage VS. Current below.

At DVs the current stops completely. If DV is less than or equal to DVs no electrons reach the collector and all electrons are repelled.
DVs is called the stopping potential.
The stopping potential is independent of the intensity of the light!
The electron is accelerated through the electric field between the collector and emitter. The energy it gains is equal to the potential energy the electron starts with. The equation for this energy is in the Electricity and Magnetism section of the equation sheet. It is given as ![]() . So at the stopping potential, the potential energy of the electrons is equal the maximum kinetic energy.
. So at the stopping potential, the potential energy of the electrons is equal the maximum kinetic energy.
We can write:
![]()
Note here that KMax is also independent of the intensity of the light!
If we look at a graph of frequency VS. kinetic energy, we see it has a straight line.
There is a minimum frequency before the electrons have any kinetic energy. The minimum frequency is called the cutoff frequency. The photons with a frequency less than fC don’t have enough energy to dislodge the electrons from the metal.
The slope of the graph is h, Planck’s constant.
The value for the cutoff frequency is simply the intercept on the x axis.
The equation for kinetic energy as a function of frequency is:
![]()
This is a linear equation and the values for it can be found from the graph.
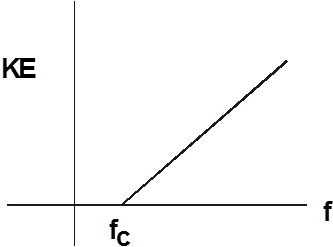
Finding the Cutoff Frequency: The cutoff frequency is the minimum frequency that will generate photoelectrons. So we use the max kinetic energy equation. The minimum frequency occurs when the kinetic energy is zero.
![]()
![]()
So the cutoff frequency is:
![]()
|
- A sodium photoelectric surface with work function 2.3 eV is illuminated by electromagnetic radiation and emits electrons. The electrons travel toward a negatively charged cathode and complete the circuit shown above. The potential difference supplied by the power supply is increased, and when it reaches 4.5 V, no electrons reach the cathode.
- For the electrons emitted from the sodium surface, calculate the following.
- The maximum kinetic energy.
![]()
![]() or
or
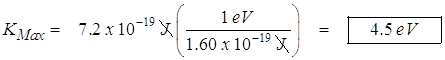
- The speed at this maximum kinetic energy.
![]()
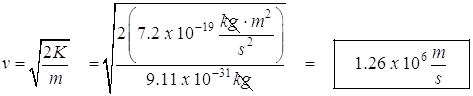
- Calculate the wavelength of the radiation that is incident on the sodium surface.

![]()
![]()
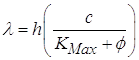

- Calculate the minimum frequency of light that will cause photoemission from this sodium surface.
![]() But
But ![]() for the minimum frequency, so
for the minimum frequency, so
![]()
![]()

- What is the cutoff wavelength for a copper metal surface?

Note that this wavelength is much smaller than visible light, so no photoelectric effect for copper with visible light – this would have to be like ultraviolet light.
Finding the Work Function: To find the work function, set the kinetic energy to zero as above and solve for f. The frequency is the cutoff wavelength, which is the minimum frequency. Recall that the work function is the minimum energy needed to break an electron out of the metal’s surface.
![]()
- 500.0 nm light is incident on a metal surface. The stopping potential is found to be 0.440 V. (a) Find the work function for this material and (b) the longest wavelength that will eject electrons from the metal.
(a) work function:
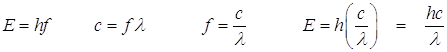
![]()
Now, when we plug in our values, we will stick in the symbol “e” for the charge of an electron. This will get us eV as a unit, eventually.

![]()
- longest wavelength
Set the maximum kinetic energy equal to zero to get the longest wavelength.

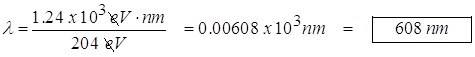
Stuff Classical Mechanics or Wave Theory Cannot Explain:
- No electrons are emitted if the light frequency falls below some cutoff frequency, fC
- Maximum kinetic energy is independent of the light intensity
- The electrons are emitted almost instantaneously
- KEMax increases with increasing frequency as it is function of hf
- Happens so fast because it is a one to one photon/electron deal
Stopping Potential versus frequency: Stopping potential is a function of frequency. The higher the frequency, the higher the stopping potential. On the following graph stopping potential is plotted along the y axis while frequency is along the x axis. As you can see, the intercept on the x axis represents the stopping potential for the cutoff frequency.
At the cutoff frequency, the total energy is equal to the work function, so
![]()
So you can find the work function if the cutoff frequency is known.
At the stopping potential, the energy of the electron is equal to the electron charge times the voltage. This is the potential energy gained by an electron in the field at the stopping potential.
![]()
But this energy has to equal the energy of the photon, so:
![]()
q is the charge of an electron, so we can plug in “e” for the electron’s charge and write the equation as:
![]()
solving for h/e

Thus, h/e is simply the slope of the graph.

AP Test Item: In a photoelectric experiment, light is incident on a metal surface. Electrons are ejected from the surface, producing a current in a circuit. A reverse potential is applied in the circuit and adjusted until the current drops to zero. That potential at which the current drops to zero is called the stopping potential. The data obtained for a range of frequencies are graphed below.
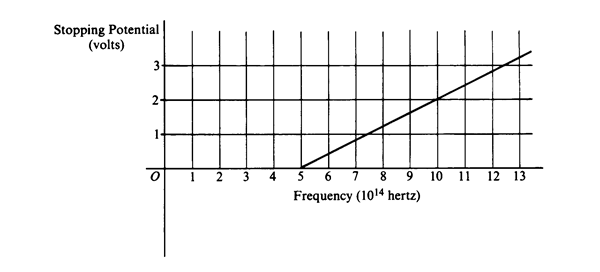 |
- For a frequency of light that has a stopping potential of 3.0 volts, what is the maximum kinetic energy of the ejected photoelectrons?
Set the maximum kinetic energy equal to the potential energy gained by the electrons in the electric field at the stopping potential.
![]()
b. From the graph and the value of the electron charge, determine an experimental value for Planck's constant.
Slope of graph is: ![]()
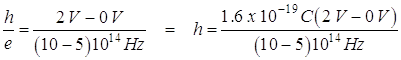
 or
or

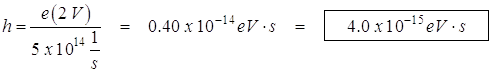
- From the graph, determine the work function for the metal.
The graph is a straight line so: ![]()
Plug in the values on the graph for the terms in the straight line equation:
![]()
But eVs is KMax ![]() so y intercept is work function f
so y intercept is work function f
This is ![]()

d. On the axes above, draw the expected graph for a different metal surface with a threshold frequency of 6.0 x 1014 hertz.
Dear Doctor Science,
What is a laser beam made of?
-- Lauren Grace from Toledo, OH
Dr. Science responds:
Normal light is comprised of zillions of photons. Laser light is made of futons, which are fat, stuffed photons with a zipper down the side. Some have a foam core and these are often mistakenly referred to as mumesons, which is just a fancy oriental term for futon. As in retail
advertising, Science often gives the prosaic a new name to make it seem like things are really happening when, in fact, everyone is just playing Tetris on their office computers and waiting for lunch.
Dear Straight Dope:
How does a particle accelerator work? I know it has something to do with huge magnets, but how can they make two atoms line up correctly and hit each other at such incredible velocities? Also how do they ensure that there are no other atoms besides the ones the are colliding inside the track?
--Ryan, Tucson, AZ
Cecil replies:
Jeez, if you're going to slam only two particles together at a time, you're going to be the absolute worst accelerator physicist ever. A particle accelerator isn't the delicate instrument you imagine--it's more akin to a fire hose. The idea is to aim billions and billions of particles at billions and billions of other particles, cross your fingers, and hope for the best. The more particles you have in the beam, and the more tightly bundled the particles are, the better chance you have that you'll get a collision that produces exciting new particles.
The first step in operating a particle accelerator is coming up with some particles to accelerate. You can get electrons from a device called an electron gun, which boils electrons off a filament. An electron gun is basically the same device you have in the back of your TV or your computer monitor. You can get protons by ionizing (that is, stripping the electrons off of) hydrogen gas, whose atoms consist of one proton plus one electron. To get other types particles, you need to smash your accelerated protons or electrons into something else in order to produce other particles, then use some fancy particle selection techniques to separate the wheat from the chaff, as it were. But we're getting ahead of ourselves.
The main tool for accelerating particles is powerful electric fields. (We'll get to the powerful magnets in a bit.) Let's say you're accelerating electrons (which are negatively charged). You turn on a powerful electric field, and the electrons rush to positive potential. As the electrons get there, you turn off the first electric field and turn on another one downstream, and the electrons rush there. You keep doing this until your electrons are rushing like blazes and you've achieved the electron speed you desire. Of course the process is a little more sophisticated than I've described. Instead of turning on and off the electric fields, you modulate your electric fields sinusoidally, such that the negative electric potential is always just behind where your electrons are, and the positive electric potential always just in front. The electrons sort of surf down the beam line on the electric field modulation wave.
Where do magnets come in? They have two purposes: to steer the beam and to focus it. To steer the beam you set up a dipole magnetic field (a dipole field is a regular uniform magnetic field; you can create one with permanent magnets, but usually you use an electromagnet). When charged particles cross a magnetic field, they experience a force perpendicular to both the magnetic field and to the particle's direction of motion (F = qv x B, for you scientist-types). Thus, if you have an up-down magnetic field, and the particles are moving forward, the particle will turn to the left or right, depending on its charge.
Obviously you need to steer your beam so that it collides with something, but there are other difficulties as well. To accelerate your particles, you need a lot of electric fields and magnets, and a lot of room for your particles to build up speed. Stanford's linear accelerator is two
miles long! That means two miles' worth of magnets, two miles' worth of beam pipe, two miles' worth of vacuum, electricity, tunnel, radiation shielding, etc. It's really huge. And impressive. If you get a chance, take a tour of the Stanford Linear Accelerator Center--they'll let you stand in the access gallery and you can look up and down the two-mile-long facility.
However, most people don't have two miles to donate to particle acceleration (and you'd need a lot more than two miles to accelerate protons, which are 2000 times heavier than electrons). Typically, you reuse your accelerator components by moving your particles around a ring accelerator, where they can pass by the same electric fields thousands of times. To steer your particles around the ring you'll need powerful magnets. The main disadvantage of a ring accelerator is that every time you steer a charged particle, it gives off energy in the form of synchrotron radiation (powerful X-rays). You have to compensate for this energy loss with more and bigger electric fields, which cost a lot to operate, and when you have X-rays being sprayed off all around your accelerator, you've got a shielding nightmare.
The other way magnets come into play is in focusing the beam. You want your particles to be in as tight a beam as possible, particularly if you're trying to slam them into another beam. A higher concentration of particles means a higher probability that a couple of them will smash directly into each other. In addition, if you try to cram a bunch of electrons together, they will start to repel each other, and naturally make the beam larger. For focusing you use quadrapole magnets. These focusing magnets have specialized magnetic fields, such that if a particle is straying to the right, the magnet steers it a little to the left, but if the particle is straying to the left, it gets steered a little to the right.
Finally you've got to steer the beam so it smashes into something. The two smashing methods are fixed target and colliding beams. For fixed target, you slam a beam of particles into some stationary chunk of matter. The advantages of the fixed target method are: (a) it's easier to aim the beam at a large non-moving target, (b) lots and lots of protons are available in chunks of matter, and (c) most all the products of the particle interaction will be moving in a very definite forward direction, so you can easily build detectors in the forward region to detect them. The disadvantages are that chunks of matter pretty much only provide protons and neutrons to smash into--the electrons aren't concentrated enough to be consequential--and there are certainly no anti-protons or positrons (anti-electrons). Also, a target which is just sitting there does not bring any kinetic energy into the equation. Since you are typically trying to convert energy into massive particles (via E = mc2), more energy is better.
That brings us to the other particle smashing method, colliding beams. For colliding beams, you typically smash particles into their antiparticles. (The HERA accelerator at DESY in Germany is unique in that it collides electrons or positrons with protons, for some very interesting physics effects.) For example, to create positrons (anti-electrons), you first accelerate some regular electrons, smash them into a fixed target, and collect any positrons resulting from this interaction. This generally takes a few minutes. Then you start accelerating the positrons and electrons. In a linear accelerator, you accelerate the positrons in a bunch just behind the electrons. (They surf the same electric field waves, but they surf up instead of down, if you get my drift.) In a ring accelerator, you're golden because the same accelerator elements that steer electrons clockwise automatically steer positrons counter clockwise.
The advantage of colliding beams is you get lots of energy from both beams, so you can create lots of exciting particles. One disadvantage is that collisions occur in the center of mass, so particle debris gets sprayed all over. That means you have to build a detector that completely surrounds the collision site (typically called a 4p detector), which is expensive. The other disadvantage is that it is very difficult to steer two beams directly into each other. Beams are typically the diameter of a human hair, and the length of a needle. You try smashing together two beams that size going 99.999% the speed of light!
Fortunately, there are talented accelerator physicists everywhere, or at any rate at these fine accelerators: Cornell Electron Storage Ring (CESR), Stanford Linear Accelerator Center (SLAC), Deutsches Elektronen-Synchrotron (DESY), Fermi National Accelerator Laboratory (Fermilab)), and European Organization for Nuclear Research (CERN).
-----Cecil Adams
Dear Cecil:
I have long been interested in the "Philadelphia Experiment," which was supposedly conducted by the U.S. Navy during World War II as one of the three "city projects."
The Manhattan Project, of course, was the development of the atomic bomb. The Philadelphia Experiment supposedly involved the use of magnetism to bend light rays and thus make objects invisible. Legends and sketchy reports have it that objects could be transported from place to place by the use of strong magnetic fields.
I grew up around Portsmouth, Virginia, and have long heard rumors that the degaussing facility at the mouth of the western branch of the Elizabeth River was the "receiver" facility for this project, and that a destroyer was briefly transported here before being returned to the Philadelphia Navy Yard.
Is this true?
--John H., Norfolk, Virginia
Cecil replies:
Right, John, another world-shattering secret that the military has managed to keep hushed up for 50 years. Betcha they store the giant magnets right next to the Roswell alien spacecraft.
Even the author of one of the better-known books about the Philadelphia Experiment has backed off on his more outrageous claims, although he still maintains an experiment of some kind did take place.
The whole thing first came to light in the mid-1950s, when someone variously identifying himself as Carlos Allende or Carl Allen wrote several strange letters to a UFO writer named Morris Jessup.
Filled with misspellings and stylistic eccentricities, the letters told of a U.S. Navy destroyer that in October 1943 had been subjected to a force field in an effort, apparently successful, to
make it invisible. Somehow the ship was also teleported from the Philadelphia Navy Yard to Norfolk, Virginia, and back, all within a matter of minutes. Unfortunately, the experiment also had the side effect of rendering half the officers and crew insane, with some of the crewmen unpredictably becoming invisible or bursting into flame years later. Since this had a decidedly negative effect on morale, the Navy halted the experiments and hushed up the whole affair. Or so the letter writer claimed.
The story was taken up by various writers over the years. But received its fullest treatment in The Philadelphia Experiment by William L. Moore with Charles Berlitz (1979). The book, which was the basis for a 1984 movie, claimed the ship involved was the U.S.S. Elbridge, but offered no hard evidence. The Navy, unsurprisingly, says it has no knowledge of any such experiment.
Cecil spoke with William Moore and found he no longer believes the Philadelphia Experiment involved invisibility or teleportation.
Instead, further research has convinced Moore it was part of an effort by the Navy to make ships radar-proof, supposedly in an effort to foil radar-guided torpedoes that the Germans were believed to be developing.
The idea was to feed a high-power, low-frequency current into the ship's hull, in effect making it into a radar antenna that would jam incoming radar.
But the initial experiment had unintended side effects on the crew, ranging from nausea to hallucinations and loss of consciousness. The hallucinations were the basis for the wild tales that later arose.
This version is a lot less implausible than the original yarn, but it's still got some holes in it.
A Navy historian sensibly points out there's no such thing as a radar-guided torpedo. Radar doesn't work underwater, something people understood even back in 1943. (The Germans used acoustic torpedoes, which homed in on the sound of a ship's engines.)
And Moore still doesn't have much documentary evidence. If it exists, both he and the Navy agree it's in the National Archives in Washington.
Moore says R&D records take up "a mile and a half of shelf space" and aren't indexed, so finding the right stuff could be a bit of a project. I might get around to it one of these days, but I dunno, I might have to wash my hair that night.
In the meantime purge yourself of any thoughts of invisible warships. Right now the only proven way to make ships disappear is budget cuts.
--CECIL ADAMS
 More Important Stuff: If you thought that this section of the handout was over, you are sadly mistaken. There’s lots more stuff for you to study, master, imprint on your brain, worry about, sweat over, and be all around concerned with. So here we go.
More Important Stuff: If you thought that this section of the handout was over, you are sadly mistaken. There’s lots more stuff for you to study, master, imprint on your brain, worry about, sweat over, and be all around concerned with. So here we go.
x-ray Production: x-rays, you will recall, are a group of electromagnetic waves, part of the old electromagnetic spectrum. But where do they come from?
One source, and this is how they were first discovered, is from bombarding a metal surface with high energy electrons. What happens is that the electron collides with one of the metal atoms. The electron has enough energy to remove one of the inner-shell electrons. When this happens, an outer-shell electron must drop down and fill the vacant energy shell. To do this, it must lose a large amount of energy, typically in excess of 105 eV. This energy is emitted in the form of a high-energy photon. Typical wavelengths would be between 0.001 and 0.1 nm. This is the x-ray region of the good old electromagnetic spectrum.
Wilhelm Roentgen (1845-1923) discovered x-rays. He basically constructed a cathode ray tube. This is a large, long glass tube that is evacuated so that there is a vacuum on the inside of the thing. TV picture tubes and computer monitors are basically cathode ray tubes. Anyway, at one end of the tube was a small filament called a cathode. When electric current was passed through the cathode, it gave off electrons. This is called thermonic emission. Anyway, these electrons are then accelerated to a high velocity with an electric field. Typical potential difference in such a tube would be around 30 000 to 100 000 V. The high velocity electrons collide with a metal target atoms and x-rays are given off.
Roentgen of course didn’t know that this would happen. What he did notice was that a phosphorescent screen several feet from his tube began to glow brightly when the tube was lit off. The glow continued even when he stuck a piece of wood between the tube and the screen. He concluded that some very penetrating type of radiation was being given off. Since he didn’t know what it was, he called them “x-rays”. x meaning “unknown”.
Many physics teachers in the past had cathode ray tubes that they would use for demonstrations, not realizing that they were producing massive amounts of x-rays.
x-rays are produced by TV tubes and computer monitors as well. The glass on the face of the tubes contain lead, which absorbs x-rays. Before they began doing this, it was dangerous to sit too close to a TV set. These days it’s okay – it only hurts your eyes.
Source : http://teachers2.wcs.edu/high/bhs/mikek/AP%20Physics%20Course%20Notes/Modern%20Physics/2%20-Quantum%20%20Info.doc
Web site link: http://teachers2.wcs.edu
Author : not indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly.
Quantum mechanics
Quantum mechanics
Quantum mechanics
This is the right place where find the answers to your questions like :
Who ? What ? When ? Where ? Why ? Which ? How ? What does Quantum mechanics mean ? Which is the meaning of Quantum mechanics?
Quantum mechanics physics notes
Alanpedia.com from 1998 year by year new sites and innovations
Main page - Disclaimer - Contact us