Atom nucleus and radioactivity
Atom nucleus and radioactivity
The following text is used only for teaching, research, scholarship, educational use and informative purpose following the fair use principles.
We thank the authors of the texts and the source web site that give us the opportunity to share their knowledge
Physics
Atom nucleus and radioactivity
In the early 1900’s the most popular model of the atom was ‘the plum pudding’ model; which assumed that the atom is composed of electrons surrounded by a soup of positive charge to balance the electron's negative charge, like negatively-charged ‘plums’ surrounded by positively-charged ‘pudding’.
Ernest Rutherford’s gold foil experiment
In 1909 the New Zealand physicist Ernest Rutherford carried out the following experiment;
He fired alpha particles at a very thin sheet of gold foil.
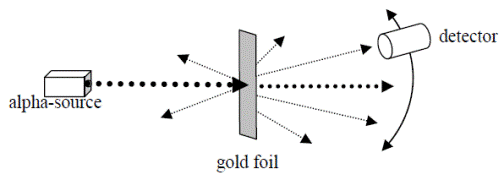
The alpha particles could be detected by small flashes of light that they produced on a fluorescent screen (see diagram).
He found that*:
- Most alpha particles were undeflected and passed straight through the gold foil.
- Some were deflected through small angles.
- A very small number were turned back through angles greater than 900!
Obviously this couldn’t be explained using the ‘plum pudding’ interpretation.
Instead Rutherford interpreted his results as follows:
- The atom is mostly empty space, but there is a solid centre, which has a positive charge.
- The electrons orbit the nucleus.
Bohr Model of the atom*
The Danish physicist Neils Bohr developed this theory to state that electrons could only inhabit certain discrete levels or orbits.
They can only gain and lose energy by jumping from one allowed orbit to another, absorbing or emitting electromagnetic radiation of frequency f, corresponding to a packet of energy of sizehf = E2 – E1 where E2 and E1 are the energies associated with the two electron levels.
Emission spectrums are lines of various colours visible when viewing a gas through a diffraction grating or spectrometer. The different colours correspond to the frequency of the electromagnetic radiation emitted.
We now know that the radius of a nucleus is about 10-15 m, while the radius of an atom is about 10-10 m*.
The atomic number (Z) of an atom tells us the number of protons present in the atom*.
The mass number (A) of an atom tells us the number of protons plus neutrons present in the atom.
Isotopes are atoms which have the same Atomic Number but different Mass Numbers.
Radioactivity
Radioactivity is the breakup of unstable nuclei with the emission of one or more types of radiation*.
However, relatively stable (and therefore non-radioactive) atoms can be made radioactive by bombarding them with neutrons.
These are known as artificial radioactive isotopes, and are often used in industry for the following;
Medical Imaging |
Food irradiation |
Radiocarbon dating |
Medical Therapy |
Agriculture |
Smoke Detectors |
Ionisation occurs when an atom loses or gains an electron.
An ion is a charged atom.
Alpha, beta and gamma radiation
The three different types of radiation emitted during radioactive decay are called Alpha, Beta and Gamma radiation.

Alpha Radiation (a)
An alpha particle is identical to a helium nucleus (2 protons and 2 neutrons).
Since they have a relatively large charge they cause a lot of ionisation as they pass through a material.
Consequently they lose their energy quickly and their penetrating ability is poor.
Charge = +2
Note that the Mass Number of the parent atom decreases by four and its Atomic Number decreases by two.
Examples:

We say that the particles on the right are ‘daughter products’.
Beta Radiation (b)
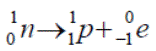 In this case a neutron splits up into a proton and an electron (and a neutrino)!!*:
In this case a neutron splits up into a proton and an electron (and a neutrino)!!*:
A beta particle is identical to a fast moving electron.
They are less ionising and therefore more penetrative than alpha particles.
 Charge = -1
Charge = -1

Examples:
Note:
The –1 below the electron symbol obviously doesn’t represent an Atomic Number; it is merely a little accounting trick used to check if the (atomic) books are balancing.
Gamma Radiation (γ)
Gamma radiation is radiation of very short wavelength (and therefore high frequency and therefore high energy (from E =hf)).
It is uncharged and so its ionising ability is relativity poor but it is highly penetrating.
There is no change in Atomic Number or Mass Number, so there is no equation as such.
Gamma radiation usually only accompanies alpha and beta decay.
Now look at Worked Problems 1, 2 and 3, page 350/1, and then try all of exercise 30.1, page 351/2*.
Half-Life
The half-life* (T1/2) of an element is the time taken for half of the nuclei in the sample to decay.
Or
The half-life (T1/2) of an element is the time taken for the activity of a sample to decrease to half of its original value.
Obviously, the more atoms that are present, the greater will be the number of disintegrations.
This is summed up by the Law of Radioactive Decay.
The law of radioactive decay states that the number of disintegrations per second is proportional to the number of nuclei present.
dN/dt = l N
Mathematically: dN/dt µ N Þ
Where N = number of nuclei present, and l is called the Decay Constant.
There is also a relationship between Half-life (T½) and the Decay Constant (l):
T½ = 0.693 / l
Now see if you can follow the graph questions on Page 353.
Maths questions
 Maths questions on radioactivity are a little like comprehension questions; you need to read the question a couple of times and then underline each relevant point of information.
Maths questions on radioactivity are a little like comprehension questions; you need to read the question a couple of times and then underline each relevant point of information.
Remember there are only two formulae: dN/dt = l N and T½ = 0.693 / l
Note that dN/dt can be expressed in any number of ways:
- ‘The activity’,
- ‘The rate of decay’,
- ‘The number of particles emitted per second’,
- ‘The number of particles undergoing decay per second’,
- ‘The number of disintegrations per second’.
The Becquerel (Bq) is the unit of activity.
One Bq = one disintegration per second.
Detecting Radiation: the Geiger-Muller Tube
Operation
Principle: A charged particle passing through a gas leaves in its wake a trail of electron-ion pairs, like a bull in a china shop. The electrons then accelerate up to the anode where they get detected as an electronic pulse.
- Radiation enters through the thin window on the left.
- It causes ionisation of some of the rare-earth gas molecules inside.
- The negative ions (electrons) accelerate towards the anode, colliding off (and ionising) other gas molecules along the way, giving rise to an avalanche effect.
- These ions all reach the anode more or less together and are detected as a pulse.
- The G-M tube may in turn be connected to a counter or loudspeaker or (in our case) both.
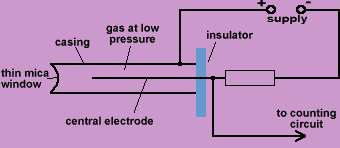
Using a G-M tube to investigate the range of Alpha, Beta and Gamma radiation in air
Or
To identify three different sources
- Get the background count.
- This is done by first setting the counter to zero without any radiation source nearby and then recording the number of counts over a 5-minute period.
- From this calculate the number of counts per second.
- Place the alpha source in front of the detector.
- Find the average count rate per second.
- Move the detector away from the source in small steps and calculate the average count rate at each step.
- Continue until count rate equals background count rate.
- Repeat for Beta source and Gamma source.
Result
The Gamma radiation will be detected at the greatest distance (from source to detector), and Alpha radiation the least.
Note
We could also have tested the penetrative ability of the different sources in a similar fashion, ie by placing different materials between source and detector.
We would find that a few sheets of paper would stop Alpha, Aluminium would be required for Beta, while lead is necessary for Gamma radiation.
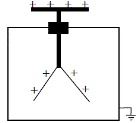
To demonstrate the ionizing affect of radioactivity
Procedure: Bring a radioactive source close to the cap of a charged Gold Leaf Electroscope
Observation: Leaves collapse
Conclusion: The charge on the G.L.E. became neutralised by the ionised air.
Radon Gas (mainly from granite rock) is the main source of background radiation, which in turn is responsible for almost all the radiation we get exposed to over our lifetime.
The effect of Ionising Radiation on humans depends on:
- The type of radiation (whether it’s alpha, beta or gamma)
- The activity of the source (in Bq)
- The time of exposure
- The type of tissue irradiated
Precautions when dealing with ionising radiation:
- Make sure sources are properly shielded.
- Keep sources as distant as possible from human contact, eg use a pair of tongs (and not, as one official safety brochure advised, a pair of thongs!).
- Use protective clothing.
Leaving Cert Physics Syllabus
Content |
Depth of Treatment |
Activities |
STS |
|
|
|
|
The Nucleus |
|
|
|
1. Structure of the atom |
Principle of Rutherford’s experiment. Emission line spectra. |
Experiment may by simulated using a large-scale model or a computer or demonstrated on a video. Demonstration of line spectra and continuous spectra. |
Lasers. |
|
|
|
|
2. Structure of the nucleus |
Atomic nucleus as protons plus neutrons. |
|
|
|
|
|
|
3. Radioactivity |
Experimental evidence for three kinds of radiation; by deflection in electric or magnetic fields or ionisation or penetration. |
Demonstration of ionisation and penetration by the radiations using any suitable method, e.g. electroscope, G-M tube. |
Uses of radioisotopes: |
|
|
|
|
|
Principle of operation of a detector of ionising radiation. Definition of Becquerel (Bq) as one disintegration per second. |
Demonstration of G-M tube or solid state detector. |
|
|
|
|
|
|
Law of radioactive decay. |
Appropriate calculations |
|
|
|
|
|
4. Nuclear Energy |
Dealt with in next chapter |
|
|
5. Ionising radiation and health hazards |
General health hazards in use of ionising radiations, e.g. X-rays, nuclear radiation; the effect of ionising radiation on humans depends on the type of radiation, the activity of the source (in Bq), the time of exposure, and the type of tissue irradiated. |
Measurement of background radiation. |
Health hazards of ionising radiation. Disposal of nuclear waste. |
Extra Credit
Some quotes:
In science there is only physics; all the rest is stamp collecting.
Lord Rutherford.
The energy produced by an atom is a very poor kind of thing. Anyone who expects a source of power from the transformation of these atoms is talking moonshine.
Rutherford.
We must be wary of using this word ‘Transmutation’ – lest people believe us to be alchemists.
When Rutherford first began splitting the atom, he was quite literally changing one element into another – the goal of Alchemists (magicians) down through the years.
I have observed many transformations in my work on radioactivity, but none so rapid as my own transformation from a physicist to a chemist”
Rutherford again, this time on receiving the Nobel Prize for Chemistry (hate that!).
Something most textbooks are uncomfortable with is the fact that the great Isaac Newton spent over 90% of his time as an Alchemist.
One noted historian claimed that Newton was not the first great scientist; he was the last of the great mystics.
*He found that . . .
Now while Rutherford was indeed a brilliant physicist, do not think that these ideas came easily to him.
For every one experiment that was productive, he had probably another 90 that were a waste of time.
See for example the video ‘Rutherford’s Atom’, available in the physics lab.
Indeed when he carried out this experiment he had no idea what the result would be. He described his astonishment at the results in very graphic terms:
“It was quite the most incredible event that ever happened to me in my life. It was as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came pack and hit you!”
Rutherford puzzled over these results for some weeks and eventually realised that the alpha particles could only be scattered through such large angles if they had collided with a very dense and small core of matter within the atom – the atomic nucleus.
*Bohr model of the atom
Shortly after 1900 the brothers Niels and Harald Bohr of Denmark became famous soccer players in Scandinavia.
In 1908 Harald won a silver medal in the first Olympic soccer competition.
Bohr was raised in a middle class Danish family and showed no particular talent as a child except for sports.
He played soccer at almost a professional level and was an active skier until late in his life.
Niels' son Aage was also a Nobel physicist.
The American actress Olivia Newton-John (Remember Saturday Night Fever anyone?) is Niels Bohr's grand-daughter.
*We now know that the radius of a nucleus is about 10-15 m, while the radius of an atom is about 10–10 m.
Therefore the radius of an atom is 100,000 times bigger than that of a nucleus.
And volume of a sphere is proportional to the cube of the radius.
This means that all matter is actually 99.99999999999 % empty space.
So if we removed all the empty space in the body, we would we left with all the mass taking up a volume about the same as a grain of sand!
Now, given that your fist is made up of atoms (which as we have seen are pretty much just empty space) why doesn’t your fist go straight through a table (which is just as empty) when you hit it?
Also, if you and I are almost completely empty space, why do we give the appearance of being solid?
AND WHY THE HELL DON’T WE DISCUSS THIS??
*The atomic number (Z) of an atom tells us the number of protons present in the atom.
Because the activity of an atom is determined by the number and arrangement of electrons, it is sometimes said that “protons give the atom its identity; electrons give it its personality”. Nice.
*Isotopes are atoms which have the same Atomic Number but different Mass Numbers.
For example Carbon-12 has 6 protons and 6 neutrons, while carbon-14 has 6 protons and 8 neutrons.
Therefore Carbon-12 and Carbon-14 are isotopes.
*Radioactivity is the disintegration of unstable nuclei with the emission of one or more types of radiation.
I think that the greater the discrepancy between the number of protons and the number of neutrons, the more radioactive an element is.
It seems that the discrepancy causes the nucleus to become unstable.
Also, the higher up the periodic table you go, the greater will be the discrepancy and therefore there is a greater likelihood that these elements will be radioactive.
So to recap; the nuclei of some atoms are unstable and as a result break up to form more stable nuclei.
These new nuclei may in turn break up further.
If we know what type of atom it is, we will be able to predict the changes which will take place within the nucleus.
But here’s the kick:
There is absolutely no way of knowing when an individual atom will decay, AND there is absolutely no way of affecting the process.
Or to put it a bit more scientifically, the decay process is unaffected by physical or chemical factors. So you can hit the atom with a kango hammer, dip it in a bath of sulphuric acid, heat it with a blow-torch or caress it softly while whispering sweet nothings in its ear – it won’t make any difference. It will decay when and only when it’s good and ready.
It is a truly random or spontaneous event (as opposed to tossing a coin for instance).
For what it’s worth, this has serious philosophical implications as it sets a limit to how much science can ever know.
So There!
*and then try all of exercise 30.1, page 351/2.
Note that in Problem 3, when trying to ascertain how many alpha and beta particles fit into a chain, you must first sort out the mass number using alpha particles, and then sort out the atomic number using beta particles.
*In this case a neutron splits up into a proton and an electron (and a neutrino)
I have to admit that I always grimace when I read this in text-books.
It’s as if this is the most natural thing in the world, like Kerry winning the All-Ireland. The phrase represents all that is wrong with physics textbooks – no wonder people think physics is boring.
Let’s take a look at this again. “A neutron splits up into a proton and an electron”. Now we know electrons do not, as a rule, live inside neutrons.
In fact they have nothing at all to do with the nucleus of an atom.
They orbit the damn thing.
AND an electron is charged, a neutron is not.
AND a neutron only has quarks in it, and quarks and electrons are completely different (it says so in the textbook).
So how/why can a neutron spit out an electron?
Now there have been some strange births in our time - there have been cases of women giving birth to a baby which in turn had a foetus inside her.
There have been reports of a woman giving birth to a child without any conception having taken place – but I’ve never, EVER heard of anything stranger than a neutron giving birth to an electron and a proton.
Maybe it’s just me.
But here’s the thing.
Once the process doesn’t break any of the laws of physics (e.g. conservation of energy, charge, momentum etc).then it’s allowed, and apparently this process doesn’t break any.
By the way;
Don’t make the mistake of assuming that the neutron is actually a proton and an electron bound together, which come apart.
That idea was rejected in the 1930s.
The Neutrino
Beta decay also includes the emission of another particle called the neutrino, which wasn’t discovered until decades later, so for some reason we ignore it in this chapter but include it when studying the Particle Physics chapter.
*Half-Life
One of many analogies for half-life is the Gold Leaf Electroscope.
They are very easily broken.
In fact, after every 40-minute class using them, approximately half of them need to be repaired.
It is (almost) impossible to predict in advance which electroscopes will break (although one could take a look at the students involved and make an educated guess from there).Assuming the broken ones do not get repaired, then the half which are still in working order get handed out in the next class.
After 40 minutes, half of these come back broken.
And so on. You could say that the half-life of a gold leaf electroscope is 40 minutes.
We can say the same about the decay of a large number of radioactive atoms (of the same element).
If the element is Radon, then after a certain time approximately half of the atoms will have decayed.
This time will be the same for Radon no matter how many atoms are present (assuming that there are a very large number). It’s a lot like saying that if I toss a coin it will come up heads half the time. This will only be accurate if we are talking about a very large number of coin tosses.
The time it takes half of the radon atoms to decay is unique to radon and is called the half-like of radon.
Each element has its own unique half-life.
Protactinium-234, for instance, has a half-life of 1.2 minutes, while Uranium-238 has a half-life of 4.5 billion years!
See the chain below for more examples.
Did you know?
Each cubic metre of garden top soil contains typically:
0.5 grams of Uranium and the members of its decay chain.
1.5 grams of Thorium and the members of its decay chain.
Brazil nuts contain small amounts of radium, a radioactive material. Although the amount is very small, about 1–7 pCi/g (40–260 Bq/kg), and most of it is not retained by the body, this is 1,000 times higher than in other foods. According to Oak Ridge Associated Universities, this is not because of elevated levels of radium in the soil, but due to "the very extensive root system of the tree."
Source: Wikipedia
A 70 kg human has about 9 kBq of natural radioactivity; mostly K-40 and C-14.
Polonium
Marie Curie discovered a new element while working on radioactivity.
At the time (circa 1900) her country was in danger of being annexed by Germany. Fearing nobody would ever remember that her country had even existed, she called the new element Polonium so we would never forget.
Her notebooks are still so radioactive that they are kept in lead cases!
Decay Chains
The daughter nuclide of a decay event may also be unstable (radioactive). In this case, it will also decay, producing radiation. The resulting second daughter nuclide may also be radioactive. This can lead to a sequence of several decay events. Eventually a stable nuclide is produced. This is called a decay chain.
An example is the natural decay chain of uranium-238 which is as follows:
decays, through alpha-emission, with a half-life of 4.5 billion years to thorium-234
which decays, through beta-emission, with a half-life of 24 days to protactinium-234
which decays, through beta-emission, with a half-life of 1.2 minutes to uranium-234
which decays, through alpha-emission, with a half-life of 240 thousand years to thorium-230
which decays, through alpha-emission, with a half-life of 77 thousand years to radium-226
which decays, through alpha-emission, with a half-life of 1.6 thousand years to radon-222
which decays, through alpha-emission, with a half-life of 3.8 days to polonium-218
which decays, through alpha-emission, with a half-life of 3.1 minutes to lead-214
which decays, through beta-emission, with a half-life of 27 minutes to bismuth-214
which decays, through beta-emission, with a half-life of 20 minutes to polonium-214
which decays, through alpha-emission, with a half-life of 160 microseconds to lead-210
which decays, through beta-emission, with a half-life of 22 years to bismuth-210
which decays, through beta-emission, with a half-life of 5 days to polonium-210
which decays, through alpha-emission, with a half-life of 140 days to lead-206, which is a stable nuclide.
Some radionuclides may have several different paths of decay. For example, approximately 36% of bismuth-212, decays, through alpha-emission, to thallium-208 while approximately 64% of bismuth-212 decays, through beta-emission, to polonium-212. Both the thallium-208 and the polonium-212 are radioactive daughter products of bismuth-212, and both decay directly to stable lead-208.
Source: Wikipedia
Exam questions
The atom

- [2002][2008 OL]
- The diagram shows a simplified arrangement of an experiment carried out early in the 20th century to investigate the structure of the atom. Name the scientist who carried out this experiment.
- Describe what was observed in this experiment.
- Why was it necessary to carry out this experiment in a vacuum?
- What conclusion did the scientist form about the structure of the atom?
- [2005]
Rutherford had bombarded gold foil with alpha particles. What conclusion did he form about the structure of the atom?
- [2009][2005][2008 OL]
What is the structure of an alpha particle?
- [2008 OL]
How are the electrons arranged in the atom?
- [2006]
Describe the Bohr model of the atom.
- [2007]
Describe how an emission line spectrum is produced.
When the gas is heated the electrons in the gas are move up to higher orbital level and as they fall back down they emit electromagnetic radiation of a specific frequency.
- [2008]
When the toaster is on, the coil emits red light.
Explain, in terms of movement of electrons, why light is emitted when a metal is heated.
- [2007][2003][2009 OL]
What is an isotope?
- [2002 OL]
Give two examples of radioisotopes.
- [2003]
How many neutrons are in a 14C nucleus?
Radioactivity
- [2003][2003 OL]
What is radioactive decay?
- [2004 OL][2005 OL][2007 OL][2010 OL]What is radioactivity?
- [2009 OL]
- Name the three types of radiation.
- Which radiation is negatively charged?
- Which radiation has the shortest range?
- Which radiation is not affected by electric fields?
- [2004 OL]
Name the French physicist who discovered radioactivity in 1896.
- [2002 OL]What is measured in becquerels?
- [2003][2002 OL][2005 OL]Apart from “carbon dating”, give two other uses of radioactive isotopes.
- [2002 OL]
Give two examples of radioisotopes.
- [2008][2007][2003 OL][2004 OL][2005 OL][2007 OL][2008 OL][2009 OL]
Name an instrument used to detect radiation/ alpha particles/ measure the activity of a sample.
- [2008][2007][2004 OL]
What is the principle of operation of this instrument?
- [2004 OL] [2005][2010 OL]
Give two uses of a radioactive source.
- [2005]
Nuclear disintegrations occur in radioactivity and in fission.
 Distinguish between radioactivity and fission.
Distinguish between radioactivity and fission.
- [2005]
Radioactivity causes ionisation in materials. What is ionisation?
- [2005]
Describe an experiment to demonstrate the ionising effect of radioactivity.
- [2004 OL]
- The diagram illustrates that three types of radiation are emitted from a radioactive source. Name the radiations labelled (i) X, (ii) Y, (iii) Z, in the diagram.
- Which one is the most ionising?
 [2010 OL]
[2010 OL] - The diagram shows a shielded radioactive source emitting nuclear radiation.
How do you know that the source is emitting three types of radiation?
- Name the radiation blocked by each material
Half-life
- [2007][2002 OL][2005 OL]
Explain the term half-life.
- [2005 OL]
Na−25 is a radioactive isotope of sodium. It has a half life of 1 minute.
What fraction of a sample of Na−25 remains after 3 minutes?
- [2007 OL]
The half life of a radioactive element is 3 days.
What fraction of a sample of the radioactive element will remain after 9 days?
- [2004]
The activity of a radioactive isotope decays to 1/16th of its original value after 36 years.
What is the half-life of the isotope?
- [2007]
An ancient wooden cup from an archaeological site has an activity of 2.1 Bq.
The corresponding activity for newly cut wood is 8.4 Bq.
If the half-life of carbon-14 is 5730 years, estimate the age of the cup.
- [2003]
14C is a radioactive isotope of carbon with a half-life of 5730 years.
How much of a 14C sample remains after 11 460 years?
- [2006]
A neutral pion is unstable with a decay constant of 2.5 × 1012 s–1. What is the half-life of a neutral pion?
- [2009]
Americium-241 has a decay constant of 5.1 × 10–11 s–1.
Calculate its half life in years.
- [2003[
14C is a radioactive isotope of carbon with a half-life of 5730 years.
Calculate the decay constant of 14C.
- [2005]
- Cobalt−60 is a radioactive isotope with a half-life of 5.26 years.
Calculate the decay constant of cobalt−60.
- Calculate the rate of decay of a sample of cobalt−60 when it has 2.5 × 1021 atoms.
- [2007]
When a tree is cut down the carbon-14 present in the wood at that time decays by beta emission.
Write a nuclear equation to represent the decay of carbon-14.
- [2003]
14C decays to 14N. Write an equation to represent this nuclear reaction.
- [2005]
Cobalt−60 is a radioactive isotope and emits beta particles.
Write an equation to represent the decay of cobalt−60.
- [2007 OL]
Read this passage and answer the questions below. Radon is a naturally occurring radioactive gas. It originates from the decay of uranium, which is present in small quantities in rocks and soils. Radon is colourless, odourless and tasteless and can only be detected using special equipment, like a Geiger-Müller tube, that can measure the radiation it releases. Because it is a gas, radon can move freely through the soil and enter the atmosphere. When radon reaches the open air, it is quickly diluted to harmless concentrations, but when it enters an enclosed space, such as a house, it can sometimes accumulate to unacceptably high concentrations. Radon can enter a building from the ground through small cracks in floors and through gaps around pipes and cables. Radon is drawn from the ground into a building because the indoor air pressure is usually lower than outdoors. Being radioactive, radon decays releasing radiation.When radon is inhaled into the lungs the radiation released can cause damage to the lung tissue.
(Adapted from Understanding Radon, A Householder’s Guide by the RPII.)
- What is the source of radon?
- How does radon enter a building?
- How can the build-up of radon in the home be prevented?
- Why is radon dangerous?
- Why is radon harmless in the open air?
- Name a radioactive element other than radon.
- [2003]
Why does the 12C in dead tissue remain “undisturbed”?
- [2002 OL]
What is meant by background radiation?
- [2010]
Name the naturally occurring radioactive gas which seeps into buildings from underground rocks and which can cause lung cancer.
- [2003 OL][2004 OL][2010 OL]
Give two precautions that are taken when storing the plutonium / dealing with radioactive sources.
- [2004 OL][2002 OL][2010 OL]
Give two effects of radiation on the human body.
- [2009]
Smoke detectors use a very small quantity of the element americium-241. This element does not exist in nature and was discovered during the Manhattan Project in 1944.
Alpha particles are produced by the americium-241 in a smoke detector.
- How are the alpha particles produced?
- Why do these alpha particles not pose a health risk?
- Explain why americium-241 does not exist naturally.
{I don’t think this was a fair question and shouldn’t have appeared on the paper}
Exam solutions
- Ernest Rutherford.
- Most alpha particles passed straight through; some were deflected by various amounts and a small percentage bounced back completely.
- To prevent the alpha particles colliding with other particles.
- It consists of a small, dense, positively charged core with negatively charged electrons circling around it.
- The atom was mostly empty space with a dense positively-charged core and with negatively-charged electrons in orbit around it.
- An alpha particle is identical to a helium nucleus (composed of 2 protons and 2 neutrons).
- They orbit the nucleus at discrete levels.
- A dense positively-charged nucleus with the negatively-charged electrons in orbit at discrete levels around it.
- When the gas is heated the electrons in the gas are move up to higher orbital level and as they fall back down they emit electromagnetic radiation of a specific frequency.
- Electrons gain energy and jump to higher energy. Then when they fall back down they emit electromagnetic radiation in the form of light.
- Isotopes are atoms which have the same atomic number but different mass numbers.
- Iodine, caesium, radon, carbon 14, etc.
- Eight
- Radioactive decay is the breakup of unstable nuclei with the emission of one or more types of radiation.
- Radioactivity is the breakup of unstable nuclei with the emission of one or more types of radiation.
- Alpha (α), beta (β) and gamma (γ).
- Beta (β)
- Alpha (α)
- Gamma (γ)
- Henri Becquerel (you shouldn’t have been asked this).
- Rate of decay, activity of a radioactive substance.
- Medical imaging, (battery of) heart pacemakers, sterilization, tracers, irradiation of food, killing cancer cells, measuring thickness, smoke detectors, nuclear fuel, detect disease, detect leaks.
- Iodine, caesium, radon, carbon 14, etc.
- Geiger Muller tube.
- Incoming radiation causes ionisation of the gas.
- Carbon dating, radiotherapy, sterilising medical equipment, killing bacteria in food, smoke alarm
- Radioactivity is the breakup of unstable nuclei with the emission of one or more types of radiation.
Nuclear Fission is the break-up of a large nucleus into two smaller nuclei with the release of energy (and neutrons).
- Ionisation occurs when a neutral atom loses or gains an electron.
- Apparatus: radioactive source and charged (gold leaf) electroscope
Procedure: bring radioactive source close to the cap
Observation: leaves collapse
Conclusion: charge leaks away through ionised air / electroscope neutralised by ionised air
- X = alpha, Y = gamma, (iii) Z = beta.
- Alpha.
- One type stopped by the paper, 2nd by the aluminium and the 3rd by the concrete.
- paper blocks alpha / α,
aluminium blocks beta/ β,
concrete blocks gamma/ γ
- Time for half the radioactive nuclei in a sample to decay
- After one minute half has decayed and half remains, after 2 minutes (2 half-lives) ¾ has decayed and ¼ remains; after 3 minutes 7/8ths has decayed and 1/8th remains.
- After 3 days (one half-life) ½ would remain, after 6 days (two half –lives) ¼ would remain, and after 9 days (three half-lives) ⅛ would remain.
- 1 → 1/2 →1/4 →1/8 → 1/16 = 4 half-lives
Answer: 9 years
- 8.4 Bq to 2.1 Bq requires two half-lives.
Answer =11,460 years
- 11,460 corresponds to two half lives, and after two half lives one quarter remains.
- T1/2 = ln 2 (= 0.693) /λ
T1/2 = 0.693 / 2.5 × 1012
T1/2 = 2.8 ×10-13 s
- T½ = 0.693 / l Þ T½ = 0.693 / 5.1 × 10–11 Þ T½ = 1.36 × 1010 seconds = 430.6 years
- T1/2 = ln 2 /λ Þ λ = 0.693/5730 = 1.21 × 10−4 y-1 = 3.8×10−12 s-1
- Formula: T1/2 = ln 2/λ Þ λ = ln 2/T1/2
T1/2 = 5.26 y = 1.66 × 108 s and ln 2 = 0.693
λ = 0.693/ 1.66 × 108 Þ λ = 4.18 × 10-9 s-1
- dN/dt = (-) λN = (4.18 × 10-9)( 2.5 × 1021) = 1.04 × 1013 Bq
- 146C → 714N + -10e ( accept e in lieu of β)
- 146C → 714N + -10e
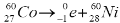
- Uranium, radium, rocks, soil.
- Through small cracks, through the floor, through gaps around pipes
- By installing a radon membrane, installing a depressurising unit, sealing cracks, sealing gaps, having good ventilation, etc.
- It can cause damage to lung tissue (it can cause cancer).
- It is diluted (to harmless concentrations)
- Uranium, radium, plutonium, carbon 14, etc.
- It is not radioactive, it is not exchanging with the atmosphere, it is stable.
- Radiation which is in the environment due to rocks/cosmic radiation.
- Radon (gas)
- Use thick shielding, use a tongs, use protective clothing, etc.
- Cancer, skin burns, sickness, cataracts, cause sterility, genetic, etc.
- α-decay is produced when the americium (which is radioactive) undergoes radioactive decay.
- They have a very short range so are either contained within the smoke detector itself or just travel a cm or two through the air.
- Its half life is very short (with respect to age of the universe) and because it is not a member of a decay series it is not produced ‘in nature’ (it is created artificially).
Source : http://www.thephysicsteacher.ie/LC%20Physics/Student%20Notes/30.%20The%20Atom,%20Nucleus%20and%20Radioactivity.doc
Web site link: http://www.thephysicsteacher.ie
Author : not indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly.
Atom nucleus and radioactivity
Atom nucleus and radioactivity
Atom nucleus and radioactivity
This is the right place where find the answers to your questions like :
Who ? What ? When ? Where ? Why ? Which ? How ? What does Atom nucleus and radioactivity mean ? Which is the meaning of Atom nucleus and radioactivity?
Atom nucleus and radioactivity physics notes
Alanpedia.com from 1998 year by year new sites and innovations
Main page - Disclaimer - Contact us