States of matter
States of matter
The following text is used only for teaching, research, scholarship, educational use and informative purpose following the fair use principles.
We thank the authors of the texts and the source web site that give us the opportunity to share their knowledge
Chemistry
States of matter
CHEMISTRY-Notes, Chapter 13: States of Matter
Section 13.1-The Nature of Gases
KEY CONCEPTS
- What are the three main assumptions of the kinetic theory as it applies to gases?
- How does kinetic theory explain gas pressure?
- What is the relationship between the temperature in kelvins and the average kinetic energy of particles?
--The KINETIC ENERGY of an object is the energy it has because of its mass and velocity
KE = ½ mv2
--KINETIC THEORY states that all matter consists of tiny particles in constant motion; for gases, it also assumes
1. Gas particles are small, hard spheres with insignificant volume, with no attractive or repulsive forces between them
2. The motion of gas particles is rapid, constant, and random
Ex. O2 molecules at 20oC move at 1700 km/h
3. All gas particle collisions are perfectly elastic (KE is transferred from one particle to another without loss; total KE remains constant)
SEE FIG. 13.1, P. 386
--GAS PRESSURE is the force exerted by a gas per unit surface area of an object
P = F/A
--Gas pressure is the result of simultaneous collisions of billions of rapidly moving particles in a gas with an object
--A VACUUM is the result when no particles are present to exert pressure
--ATMOSPHERIC PRESSURE results from collisions of particles in air with objects
--Why does atmospheric pressure decrease with altitude?
--A BAROMETER is a device used to measure atmospheric pressure
SEE FIG. 13.2

--Note that atmospheric pressure can be measured as the height of a column of mercury atmospheric pressure will support
--The SI unit of pressure is a PASCAL (Pa);
1 Pa = 1N/m2
Since a pascal is so small, kPa are more commonly used for measuring atmospheric pressure
--STANDARD ATMOSPHERIC PRESSURE is average atmospheric pressure measured at sea level
1 atmosphere (atm) = 760 mm Hg = 760 torr =
101.3 kPa = 14.7 psi
Ex. Convert 35 psi to kPa
--A MANOMETER is a device used to measure gas pressure; two types
1. Closed-end manometer

Here, the pressure of the gas is the difference in height in the two sides of the tube
2. Open-end manometer

In this case, is the pressure of the gas more or less than atmospheric pressure? How much more or less?
--Recall that temperature is a measure of the average kinetic energy of particles
Ex. the particles in a sample of iron, water, and helium at the same temperature have the same kinetic energy
--What is ABSOLUTE ZERO?
--The Kelvin temperature of a substance is directly proportional to the average kinetic energy of the particles of the substance
Ex. How does the average kinetic energy of helium atoms at 200 K compare to their average KE at 100 K?
SEE 13.1 SECTION ASSESSMENT
Section 13.2-The Nature of Liquids
KEY CONCEPTS
- What factors determine the physical properties of a liquid?
- What is the relationship between evaporation and kinetic energy?
- When can a dynamic equilibrium exist between a liquid and its vapor
- Under what conditions does boiling occur?
--A FLUID is any substance that can flow
Ex. liquids and gases are fluids and conform to the shape of their containers
SEE FIG. 13.5, P. 390
--However, unlike in gases, the particles of a liquid are attracted to each other, resulting in a definite volume for a liquid
--The interplay between the disruptive motions of particles in a liquid and the attractions among the particles determines the physical properties of liquids
--CONDENSED STATES of matter (solids and liquids) are virtually incompressible because interparticle attractions reduce the amount of space between particles
--VAPORIZATION is the conversion of a liquid to a gas (vapor-gaseous state of a substance that is normally a solid or liquid at room temperature)
--EVAPORATION is vaporization that takes place at the surface of a liquid that is not boiling
--During evaporation, only molecules with a certain minimum KE can escape the surface of the liquid
--What happens to the average KE of the liquid left behind as evaporation takes place? Temperature?
--How does increasing temperature of a liquid affect the rate of evaporation? Why does it have this effect?
--If an open container of liquid is left to evaporate, eventually all the liquid will evaporate. However, if it is closed, the particles cannot escape. As more vapor accumulates, some of the particles will condense back and return to the liquid state
SEE FIG. 13.6, P. 391
--A LIQUID-VAPOR DYNAMIC EQUILIBRIUM is established when the rates of evaporation and condensation are equal, resulting in no net change in the amount of liquid or vapor
--VAPOR PRESSURE is the pressure exerted by a vapor when it is in equilibrium with a condensed state; is temperature dependent
SEE TABLE 13.1, 13.2, PP. 392-393
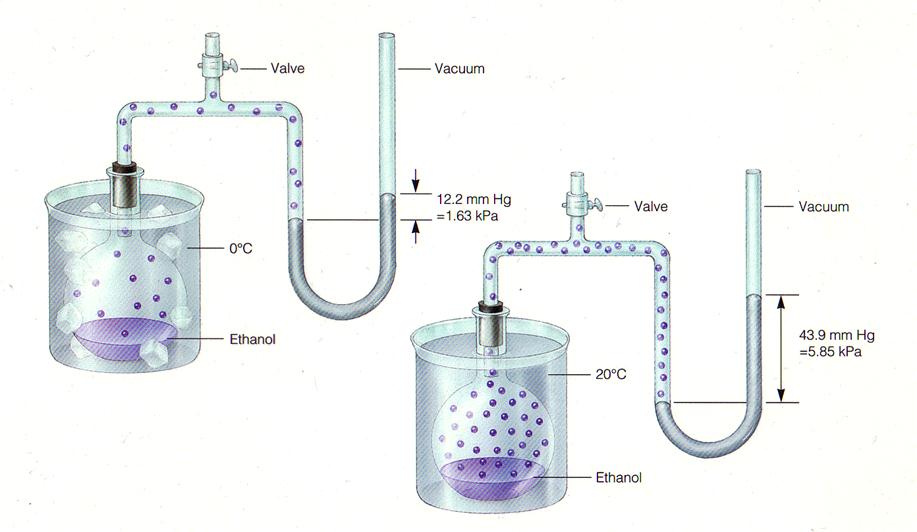
--What relationship (if any) exists between the vapor pressure of a liquid or solid and interparticle forces? VOLATILITY (ease of evaporation) and interparticle forces?
--When a liquid is at a temperature at which particles throughout the liquid have enough kinetic energy to vaporize, the liquid will boil
--The BOILING POINT of a liquid is the temperature at which the vapor pressure of the liquid is equal to the external pressure on the liquid
Ex. alcohol demonstration
--In what two ways are boiling and evaporation different?
--What happens to the boiling point of a liquid if the external pressure on it is lowered? If pressure is increased?
SEE FIG. 13.8, P. 394
--The NORMAL BOILING POINT of a liquid is the temperature at which the liquid will boil under standard pressure
SEE FIG. 13.9, TABLE 13.2, PP. 394-395
--Note that the temperature of a boiling liquid cannot rise above its boiling point. More heat only raises the number of particles capable of escaping the liquid
--What is the difference between water molecules at 100oC and water vapor molecules at 100oC, besides different states?
SEE 13.2 SECTION ASSESSMENT, P. 395
Section 13.3-The Nature of Solids
KEY CONCEPTS
- How are the structure and properties of solids related?
- What determines the shape of a crystal?
--The general properties of a solid reflects the orderly arrangements and fixed locations
--The MELTING POINT of a solid is the temperature at which the organization of particles within the solid breaks down, the vibrations of particles are strong enough to overcome attractions holding them in fixed positions, and the solid changes to a liquid
--Note that the melting and freezing point of a pure substance is the same temperature
--At the melting/freezing point, the liquid and solid phases are in equilibrium. What does this mean?
--Most solids are crystalline; in a CRYSTAL the particles are arranged in an orderly, repeating, three-dimensional pattern called a CRYSTAL LATTICE
--Melting point is generally determined by the type of bonding between particles:
1. Ionic compounds-high m.p.
2. Covalent compounds-low m.p.
--Crystals are classified into seven groups or CRYSTAL SYSTEMS, depending on the angles between the faces of the crystal
SEE FIG. 13.11, P. 397
--A UNIT CELL is the smallest group of particles within a crystal that retains the geometric shape of the crystal
--ALLOTROPES are two or more different molecular forms of the same element in the same physical state
Ex. carbon has 3 common allotropes-diamond, graphite, and carbon black (soot)
Ex. oxygen-O2 and O3 (ozone)
--An AMORPHOUS SOLID lacks an ordered internal structure; atoms are randomly arranged
Ex. glass is sometimes called a supercooled liquid
SEE 13.3 SECTION ASSESSMENT, P. 399
Section 13.4-Changes of State
KEY CONCEPTS
- When can sublimation occur?
- How are the conditions at which phases are in equilibrium represented on a phase diagram?
--SUBLIMATION is the change of a substance from solid to gaseous state without passing through the liquid state
Ex. dry ice, moth balls, freezer-burned meat, iodine
SEE FIG. 13.14, P. 401
--Sublimation occurs in solids with vapor pressures that exceed atmospheric pressure at or near room temperature
--DEPOSITION is the change of a substance from gaseous to solid state without condensing to a liquid
Ex. snow, frost
--A PHASE DIAGRAM indicates the state of a substance at various temperature and pressures
SEE FIG. 13.15, P. 403

--The lines separating states indicate the T and P at which those states exist at equilibrium
--The TRIPLE POINT is the T and P at which all three states can exist at equilibrium
SEE FIG. 13.16, P. 403
--Note that in the diagram above, the line separating liquid and gaseous states is vertical above point D. That point is the CRITICAL POINT
--The CRITICAL TEMPERATURE, the temperature at the critical point, is the highest temperature the substance can exist as a liquid
--CRITICAL PRESSURE is the pressure required to liquefy a substance at its critical temperature
--What are critical temperature and pressure for H2O? Normal melting and boiling point?
Source : http://www.duplinschools.net/22032033115139260/lib/22032033115139260/_files/Chemistry-Notes_Chapter_13_(States_of_Matter).doc
Web site link: http://www.duplinschools.net/
Author : not indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly.
States of matter
States of matter
States of matter
This is the right place where find the answers to your questions like :
What is States of matter? What does States of matter mean ? Which is the meaning of States of matter?
States of matter chemistry notes
Alanpedia.com from 1998 year by year new sites and innovations
Main page - Disclaimer - Contact us